beta8 integrins are required for vascular morphogenesis in mouse embryos
- PMID: 12050137
- PMCID: PMC2710098
- DOI: 10.1242/dev.129.12.2891
beta8 integrins are required for vascular morphogenesis in mouse embryos
Abstract
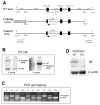
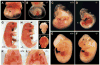
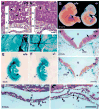
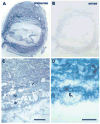
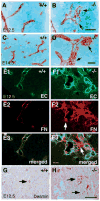
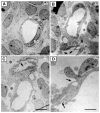
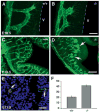
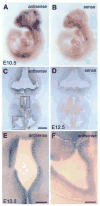
In order to assess the in vivo function of integrins containing the beta8 subunit, we have generated integrin beta8-deficient mice. Ablation of beta8 results in embryonic or perinatal lethality with profound defects in vascular development. Sixty-five percent of integrin beta8-deficient embryos die at midgestation, with evidence of insufficient vascularization of the placenta and yolk sac. The remaining 35% die shortly after birth with extensive intracerebral hemorrhage. Examination of brain tissue from integrin beta8-deficient embryos reveals abnormal vascular morphogenesis resulting in distended and leaky capillary vessels, as well as aberrant brain capillary patterning. In addition, endothelial cell hyperplasia is found in these mutant brains. Expression studies show that integrin beta8 transcripts are localized in endodermal cells surrounding endothelium in the yolk sac and in periventricular cells of the neuroepithelium in the brain. We propose that integrin beta8 is required for vascular morphogenesis by providing proper cues for capillary growth in both yolk sac and embryonic brain. This study thus identifies a molecule crucial for vascular patterning in embryonic yolk sac and brain.
Figures

Similar articles
-
Vascular development of the brain requires beta8 integrin expression in the neuroepithelium.J Neurosci. 2005 Oct 26;25(43):9940-8. doi: 10.1523/JNEUROSCI.3467-05.2005. J Neurosci. 2005. PMID: 16251442 Free PMC article.
-
Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch.Am J Pathol. 2005 Jun;166(6):1883-94. doi: 10.1016/s0002-9440(10)62497-2. Am J Pathol. 2005. PMID: 15920172 Free PMC article.
-
Beta8 integrin regulates neurogenesis and neurovascular homeostasis in the adult brain.J Cell Sci. 2009 Jun 1;122(Pt 11):1842-51. doi: 10.1242/jcs.043257. J Cell Sci. 2009. PMID: 19461074 Free PMC article.
-
Integrins in vascular development.Circ Res. 2001 Sep 28;89(7):566-72. doi: 10.1161/hh1901.097747. Circ Res. 2001. PMID: 11577021 Review.
-
Morphogenesis of the first blood vessels.Ann N Y Acad Sci. 1998 Oct 23;857:155-79. doi: 10.1111/j.1749-6632.1998.tb10115.x. Ann N Y Acad Sci. 1998. PMID: 9917840 Review.
Cited by
-
Neural Regulation of CNS Angiogenesis During Development.Front Biol (Beijing). 2015 Feb;10(1):61-73. doi: 10.1007/s11515-014-1331-y. Front Biol (Beijing). 2015. PMID: 25691893 Free PMC article.
-
The role of pericytic laminin in blood brain barrier integrity maintenance.Sci Rep. 2016 Nov 3;6:36450. doi: 10.1038/srep36450. Sci Rep. 2016. PMID: 27808256 Free PMC article.
-
Aloe Vera/Collagen Mixture Induces Integrin α1β1 and PECAM-1 Genes Expression in Human Adipose-Derived Stem Cells.Adv Pharm Bull. 2019 Oct;9(4):662-667. doi: 10.15171/apb.2019.077. Epub 2019 Oct 24. Adv Pharm Bull. 2019. PMID: 31857972 Free PMC article.
-
Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice.Nature. 2007 Sep 20;449(7160):361-5. doi: 10.1038/nature06110. Epub 2007 Aug 12. Nature. 2007. PMID: 17694047 Free PMC article.
-
Cell biology of embryonic migration.Birth Defects Res C Embryo Today. 2008 Jun;84(2):102-22. doi: 10.1002/bdrc.20125. Birth Defects Res C Embryo Today. 2008. PMID: 18546335 Free PMC article. Review.
References
-
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. - PubMed
-
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. - PubMed
-
- Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, et al. Platelet endothelial cell adhesion molecule-1 (PECAM- 1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. - PubMed
-
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

