The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain
- PMID: 12034898
- PMCID: PMC150608
- DOI: 10.1105/tpc.000620
The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain
Abstract
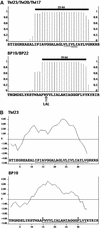
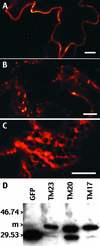
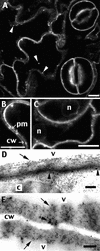
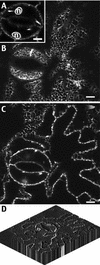
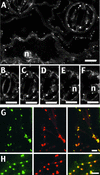
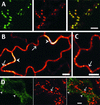
The tonoplast was proposed as a default destination of membrane-bound proteins without specific targeting signals. To investigate the nature of this targeting, we created type I fusion proteins with green fluorescent protein followed by the transmembrane domain of the human lysosomal protein LAMP1. We varied the length of the transmembrane domain from 23 to either 20 or 17 amino acids by deletion within the hydrophobic domain. The resulting chimeras, called TM23, TM20, and TM17, were expressed either transiently or stably in tobacco. TM23 clearly accumulated in the plasmalemma, as confirmed by immunoelectron microscopy. In contrast, TM17 clearly was retained in the endoplasmic reticulum, and TM20 accumulated in small mobile structures. The nature of the TM20-labeled compartments was investigated by coexpression with a marker localized mainly in the Golgi apparatus, AtERD2, fused to a yellow fluorescent protein. The strict colocalization of both fluorescent proteins indicated that TM20 accumulated in the Golgi apparatus. To further test the default destination of type I membrane proteins, green fluorescent protein was fused to the 19-amino acid transmembrane domain of the plant vacuolar sorting receptor BP-80. The resulting chimera also accumulated in the Golgi instead of in post-Golgi compartments, where native BP-80 localized. Additionally, when the transmembrane domain of BP-80 was lengthened to 22 amino acids, the reporter escaped the Golgi and accumulated in the plasma membrane. Thus, the tonoplast apparently is not a favored default destination for type I membrane proteins in plants. Moreover, the target membrane where the chimera concentrates is not unique and depends at least in part on the length of the membrane-spanning domain.
Figures
Similar articles
-
A Rab-E GTPase mutant acts downstream of the Rab-D subclass in biosynthetic membrane traffic to the plasma membrane in tobacco leaf epidermis.Plant Cell. 2005 Jul;17(7):2020-36. doi: 10.1105/tpc.105.031112. Epub 2005 Jun 21. Plant Cell. 2005. PMID: 15972698 Free PMC article.
-
Integral membrane protein sorting to vacuoles in plant cells: evidence for two pathways.J Cell Biol. 1998 Nov 30;143(5):1183-99. doi: 10.1083/jcb.143.5.1183. J Cell Biol. 1998. PMID: 9832548 Free PMC article.
-
Arabidopsis senescence-associated protein DMP1 is involved in membrane remodeling of the ER and tonoplast.BMC Plant Biol. 2012 Apr 24;12:54. doi: 10.1186/1471-2229-12-54. BMC Plant Biol. 2012. PMID: 22530652 Free PMC article.
-
Targeting of tonoplast proteins to the vacuole.Plant Sci. 2013 Oct;211:132-6. doi: 10.1016/j.plantsci.2013.07.005. Epub 2013 Jul 18. Plant Sci. 2013. PMID: 23987818 Review.
-
Organization of transport from endoplasmic reticulum to Golgi in higher plants.Biochem Soc Trans. 2000;28(4):505-12. Biochem Soc Trans. 2000. PMID: 10961949 Review.
Cited by
-
Coupled transport of Arabidopsis p24 proteins at the ER-Golgi interface.J Exp Bot. 2012 Jun;63(11):4243-61. doi: 10.1093/jxb/ers112. Epub 2012 May 10. J Exp Bot. 2012. PMID: 22577184 Free PMC article.
-
Roles of N-terminal fatty acid acylations in membrane compartment partitioning: Arabidopsis h-type thioredoxins as a case study.Plant Cell. 2013 Mar;25(3):1056-77. doi: 10.1105/tpc.112.106849. Epub 2013 Mar 29. Plant Cell. 2013. PMID: 23543785 Free PMC article.
-
Recruitment of Arf1-GDP to Golgi by Glo3p-type ArfGAPs is crucial for golgi maintenance and plant growth.Plant Physiol. 2013 Feb;161(2):676-91. doi: 10.1104/pp.112.209148. Epub 2012 Dec 24. Plant Physiol. 2013. PMID: 23266962 Free PMC article.
-
Comparison of membrane targeting strategies for the accumulation of the human immunodeficiency virus p24 protein in transgenic tobacco.Int J Mol Sci. 2013 Jun 26;14(7):13241-65. doi: 10.3390/ijms140713241. Int J Mol Sci. 2013. PMID: 23803657 Free PMC article.
-
The secreted plant N-glycoproteome and associated secretory pathways.Front Plant Sci. 2012 Jun 6;3:117. doi: 10.3389/fpls.2012.00117. eCollection 2012. Front Plant Sci. 2012. PMID: 22685447 Free PMC article.
References
-
- Ahmed, S.U., Rojo, E., Kovaleva, V., Venkataraman, S., Dombrowski, J.E., Matsuoka, K., and Raikhel, N.V. (2000). The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J. Cell Biol. 149, 1335–1344. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

