The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho
- PMID: 12011108
- PMCID: PMC2173856
- DOI: 10.1083/jcb.200202010
The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho
Abstract
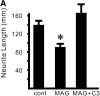
Myelin-associated glycoprotein (MAG) is a potent inhibitor of neurite outgrowth from a variety of neurons. The receptor for MAG or signals that elicit morphological changes in neurons remained to be established. Here we show that the neurotrophin receptor p75 (p75(NTR)) is the signal transducing element for MAG. Adult dorsal root ganglion neurons or postnatal cerebellar neurons from mice carrying a mutation in the p75(NTR) gene are insensitive to MAG with regard to neurite outgrowth. MAG activates small GTPase RhoA, leading to retarded outgrowth when p75(NTR)) is present. Colocalization of p75(NTR) and MAG binding is seen in neurons. Ganglioside GT1b, which is one of the binding partners of MAG, specifically associates with p75(NTR). Thus, p75(NTR) and GT1b may form a receptor complex for MAG to transmit the inhibitory signals in neurons.
Figures







Similar articles
-
Binding of soluble myelin-associated glycoprotein to specific gangliosides induces the association of p75NTR to lipid rafts and signal transduction.J Neurochem. 2005 Jul;94(1):15-21. doi: 10.1111/j.1471-4159.2005.03121.x. J Neurochem. 2005. PMID: 15953345
-
The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI.Nat Neurosci. 2003 May;6(5):461-7. doi: 10.1038/nn1045. Nat Neurosci. 2003. PMID: 12692556
-
Rap1 is involved in the signal transduction of myelin-associated glycoprotein.Cell Death Differ. 2008 Feb;15(2):408-19. doi: 10.1038/sj.cdd.4402278. Epub 2007 Nov 30. Cell Death Differ. 2008. PMID: 18049479
-
p75 neurotrophin receptor signaling in the nervous system.Biotechnol Annu Rev. 2004;10:123-49. doi: 10.1016/S1387-2656(04)10005-7. Biotechnol Annu Rev. 2004. PMID: 15504705 Review.
-
Overcoming inhibitors in myelin to promote axonal regeneration.J Neurol Sci. 2005 Jun 15;233(1-2):43-7. doi: 10.1016/j.jns.2005.03.023. Epub 2005 Apr 20. J Neurol Sci. 2005. PMID: 15949495 Review.
Cited by
-
Olfactomedin 1 interacts with the Nogo A receptor complex to regulate axon growth.J Biol Chem. 2012 Oct 26;287(44):37171-84. doi: 10.1074/jbc.M112.389916. Epub 2012 Aug 24. J Biol Chem. 2012. PMID: 22923615 Free PMC article.
-
Brain gangliosides in axon-myelin stability and axon regeneration.FEBS Lett. 2010 May 3;584(9):1741-7. doi: 10.1016/j.febslet.2009.10.011. Epub 2009 Oct 12. FEBS Lett. 2010. PMID: 19822144 Free PMC article. Review.
-
p75NTR and DR6 Regulate Distinct Phases of Axon Degeneration Demarcated by Spheroid Rupture.J Neurosci. 2019 Nov 27;39(48):9503-9520. doi: 10.1523/JNEUROSCI.1867-19.2019. Epub 2019 Oct 18. J Neurosci. 2019. PMID: 31628183 Free PMC article.
-
Bex1 is involved in the regeneration of axons after injury.J Neurochem. 2010 Nov;115(4):910-20. doi: 10.1111/j.1471-4159.2010.06960.x. Epub 2010 Sep 28. J Neurochem. 2010. PMID: 20731761 Free PMC article.
-
Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system.J Cell Biol. 2003 Jul 21;162(2):233-43. doi: 10.1083/jcb.200301080. Epub 2003 Jul 14. J Cell Biol. 2003. PMID: 12860969 Free PMC article.
References
-
- Borasio, G.D., J. John, A. Wittinghofer, Y.A. Barde, M. Sendtner, and R. Heumann. 1989. ras p21 protein promotes survival and fiber outgrowth of cultured embryonic neurons. Neuron. 2:1087–1096. - PubMed
-
- Cai, D., Y. Shen, M. De Bellard, S. Tang, and M.T. Filbin. 1999. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 22:89–101. - PubMed
-
- Davies, A.M. 2000. Neurotrophins: neurotrophic modulation of neurite growth. Curr. Biol. 10:R198–R200. - PubMed
-
- Ernfors, P., F. Hallbook, T. Ebendal, E.M. Shooter, M.J. Radeke, T.P. Misko, and H. Persson. 1988. Developmental and regional expression of beta-nerve growth factor receptor mRNA in the chick and rat. Neuron. 1:983–996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

