The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative
- PMID: 11983871
- PMCID: PMC124464
- DOI: 10.1073/pnas.102483199
The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative
Abstract
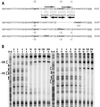
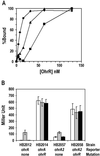
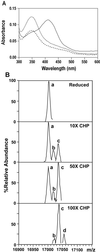
Reactive oxygen species induce the expression of detoxification and repair genes critical for life in an aerobic environment. Bacterial factors that sense reactive oxygen species use either thiol-disulfide exchange reactions (OxyR, RsrA) or redox labile 2Fe-2S clusters (SoxR). We demonstrate that the reduced form of Bacillus subtilis OhrR binds cooperatively to two adjacent inverted repeat sequences in the ohrA control region and thereby represses transcription. In the presence of organic hydroperoxides, OhrR is inactivated by the reversible oxidation of a single conserved cysteine residue to the corresponding cysteine-sulfenic acid, and perhaps to higher oxidation states.
Figures


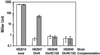
Similar articles
-
Oxidant-dependent switching between reversible and sacrificial oxidation pathways for Bacillus subtilis OhrR.Mol Microbiol. 2008 May;68(4):978-86. doi: 10.1111/j.1365-2958.2008.06200.x. Epub 2008 Mar 19. Mol Microbiol. 2008. PMID: 18363800
-
OhrR, a transcription repressor that senses and responds to changes in organic peroxide levels in Xanthomonas campestris pv. phaseoli.Mol Microbiol. 2002 Sep;45(6):1647-54. doi: 10.1046/j.1365-2958.2002.03116.x. Mol Microbiol. 2002. PMID: 12354231
-
A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR.Proc Natl Acad Sci U S A. 2007 May 22;104(21):8743-8. doi: 10.1073/pnas.0702081104. Epub 2007 May 14. Proc Natl Acad Sci U S A. 2007. PMID: 17502599 Free PMC article.
-
PerR vs OhrR: selective peroxide sensing in Bacillus subtilis.Mol Biosyst. 2010 Feb;6(2):316-23. doi: 10.1039/b915042k. Epub 2009 Sep 18. Mol Biosyst. 2010. PMID: 20094649 Review.
-
Regulation of inducible peroxide stress responses.Mol Microbiol. 2002 Jul;45(1):9-15. doi: 10.1046/j.1365-2958.2002.03015.x. Mol Microbiol. 2002. PMID: 12100544 Review.
Cited by
-
Regulation of MntH by a dual Mn(II)- and Fe(II)-dependent transcriptional repressor (DR2539) in Deinococcus radiodurans.PLoS One. 2012;7(4):e35057. doi: 10.1371/journal.pone.0035057. Epub 2012 Apr 16. PLoS One. 2012. PMID: 22523570 Free PMC article.
-
Chemical biology approaches to study protein cysteine sulfenylation.Biopolymers. 2014 Feb;101(2):165-72. doi: 10.1002/bip.22255. Biopolymers. 2014. PMID: 23576224 Free PMC article. Review.
-
Cys303 in the histidine kinase PhoR is crucial for the phosphotransfer reaction in the PhoPR two-component system in Bacillus subtilis.J Bacteriol. 2007 Jan;189(2):410-21. doi: 10.1128/JB.01205-06. Epub 2006 Nov 3. J Bacteriol. 2007. PMID: 17085571 Free PMC article.
-
Differential gene expression in response to hydrogen peroxide and the putative PerR regulon of Synechocystis sp. strain PCC 6803.J Bacteriol. 2004 Jun;186(11):3331-45. doi: 10.1128/JB.186.11.3331-3345.2004. J Bacteriol. 2004. PMID: 15150218 Free PMC article.
-
Measurement of protein sulfenic acid content.Curr Protoc Toxicol. 2008 Nov;Chapter 17:Unit17.2. doi: 10.1002/0471140856.tx1702s38. Curr Protoc Toxicol. 2008. PMID: 20963754 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

