HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells
- PMID: 11956293
- PMCID: PMC2193699
- DOI: 10.1084/jem.20012039
HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells
Abstract
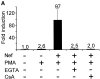
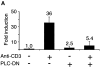
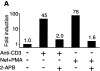
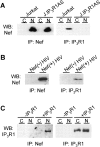
HIV-1 pathogenicity factor Nef has been shown to modulate calcium signaling in host cells, but the underlying molecular mechanisms have remained unclear. Here we show that calcium/calcineurin-dependent activation of nuclear factor of activated T cells (NFAT) by Nef in Jurkat T cells requires the endoplasmic reticulum-resident inositol trisphosphate receptor (IP(3)R), but yet does not involve increase in phospholipase-C gamma 1 (PLC gamma 1)-catalyzed production of IP(3) or depletion of IP(3)-regulated intracellular calcium stores. Nef could be coprecipitated with endogenous IP(3)R type-1 (IP(3)R1) from Nef-transfected Jurkat T cells as well as from HIV-infected primary human peripheral mononuclear cells. Thus, the Nef/IP(3)R1-interaction defines a novel T cell receptor-independent mechanism by which Nef can promote T cell activation, and appears to involve atypical IP(3)R-triggered activation of plasma membrane calcium influx channels in a manner that is uncoupled from depletion of intracellular calcium stores.
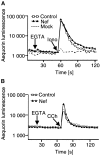
Figures




Similar articles
-
The hepatitis C virus core protein modulates T cell responses by inducing spontaneous and altering T-cell receptor-triggered Ca2+ oscillations.J Biol Chem. 2003 May 23;278(21):18877-83. doi: 10.1074/jbc.M300185200. Epub 2003 Mar 13. J Biol Chem. 2003. PMID: 12639962
-
Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway.J Biol Chem. 2000 Jun 2;275(22):16513-7. doi: 10.1074/jbc.M910032199. J Biol Chem. 2000. PMID: 10748182
-
Regulation of T cell activation by HIV-1 accessory proteins: Vpr acts via distinct mechanisms to cooperate with Nef in NFAT-directed gene expression and to promote transactivation by CREB.Virology. 2003 May 25;310(1):190-6. doi: 10.1016/s0042-6822(03)00164-8. Virology. 2003. PMID: 12788643
-
Calcium signaling mechanisms in T lymphocytes.Annu Rev Immunol. 2001;19:497-521. doi: 10.1146/annurev.immunol.19.1.497. Annu Rev Immunol. 2001. PMID: 11244045 Review.
-
Reciprocal regulation of the nuclear factor of activated T cells and HIV-1.Genes Immun. 2004 May;5(3):158-67. doi: 10.1038/sj.gene.6364047. Genes Immun. 2004. PMID: 14762397 Review.
Cited by
-
Endothelial cells promote human immunodeficiency virus replication in nondividing memory T cells via Nef-, Vpr-, and T-cell receptor-dependent activation of NFAT.J Virol. 2005 Sep;79(17):11194-204. doi: 10.1128/JVI.79.17.11194-11204.2005. J Virol. 2005. PMID: 16103171 Free PMC article.
-
Determinants of increased replicative capacity of serially passaged simian immunodeficiency virus with nef deleted in rhesus monkeys.J Virol. 2003 Jun;77(12):6823-35. doi: 10.1128/jvi.77.12.6823-6835.2003. J Virol. 2003. PMID: 12768002 Free PMC article.
-
HIV infection of astrocytes compromises inter-organelle interactions and inositol phosphate metabolism: A potential mechanism of bystander damage and viral reservoir survival.Prog Neurobiol. 2021 Nov;206:102157. doi: 10.1016/j.pneurobio.2021.102157. Epub 2021 Aug 26. Prog Neurobiol. 2021. PMID: 34455020 Free PMC article.
-
Calcium-dependent enhancement of transcription of p300 by human T-lymphotropic type 1 p12I.Virology. 2006 Sep 30;353(2):247-57. doi: 10.1016/j.virol.2006.06.005. Epub 2006 Jul 14. Virology. 2006. PMID: 16843515 Free PMC article.
-
HIV-1 Nef Impairs the Formation of Calcium Membrane Territories Controlling the Signaling Nanoarchitecture at the Immunological Synapse.J Immunol. 2016 Nov 15;197(10):4042-4052. doi: 10.4049/jimmunol.1601132. Epub 2016 Oct 19. J Immunol. 2016. PMID: 27798165 Free PMC article.
References
-
- Piguet, V., and D. Trono. 1999. The Nef protein of primate lentiviruses. Rev. Med. Virol. 9:111–120. - PubMed
-
- Skowronski, J., M.E. Greenberg, M. Lock, R. Mariani, S. Salghetti, T. Swigut, and A.J. Iafrate. 1999. HIV and SIV Nef modulate signal transduction and protein sorting in T cells. Cold Spring Harb. Symp. Quant. Biol. 64:453–463. - PubMed
-
- Renkema, G.H., and K. Saksela. 2000. Interactions of HIV-1 Nef with cellular signal transducing proteins. Front. Biosci. 5:D268–D283. - PubMed
-
- Nabel, G., and D. Baltimore. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 326:711–713. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

