Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton
- PMID: 11940607
- PMCID: PMC2199242
- DOI: 10.1083/jcb.200112113
Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton
Abstract
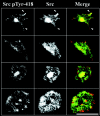
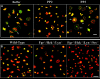
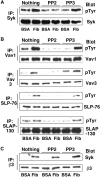
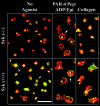
Integrins regulate cell adhesion and motility through tyrosine kinases, but initiation of this process is poorly understood. We find here that Src associates constitutively with integrin alphaIIbbeta3 in platelets. Platelet adhesion to fibrinogen caused a rapid increase in alphaIIbbeta3-associated Src activity, and active Src localized to filopodia and cell edges. Csk, which negatively regulates Src by phosphorylating Tyr-529, was also constitutively associated with alphaIIbbeta3. However, fibrinogen binding caused Csk to dissociate from alphaIIbbeta3, concomitant with dephosphorylation of Src Tyr-529 and phosphorylation of Src activation loop Tyr-418. In contrast to the behavior of Src and Csk, Syk was associated with alphaIIbbeta3 only after fibrinogen binding. Platelets multiply deficient in Src, Hck, Fgr, and Lyn, or normal platelets treated with Src kinase inhibitors failed to spread on fibrinogen. Inhibition of Src kinases blocked Syk activation and inhibited phosphorylation of Syk substrates (Vav1, Vav3, SLP-76) implicated in cytoskeletal regulation. Syk-deficient platelets exhibited Src activation upon adhesion to fibrinogen, but no spreading or phosphorylation of Vav1, Vav3, and SLP-76. These studies establish that platelet spreading on fibrinogen requires sequential activation of Src and Syk in proximity to alphaIIbbeta3, thus providing a paradigm for initiation of integrin signaling to the actin cytoskeleton.
Figures






Similar articles
-
The molecular adapter SLP-76 relays signals from platelet integrin alphaIIbbeta3 to the actin cytoskeleton.J Biol Chem. 2001 Feb 23;276(8):5916-23. doi: 10.1074/jbc.M010639200. Epub 2000 Dec 11. J Biol Chem. 2001. PMID: 11113155
-
Genetic and pharmacological analyses of Syk function in alphaIIbbeta3 signaling in platelets.Blood. 1999 Apr 15;93(8):2645-52. Blood. 1999. PMID: 10194444
-
Platelet alpha IIb-beta 3 integrin engagement induces the tyrosine phosphorylation of Cbl and its association with phosphoinositide 3-kinase and Syk.Biochem J. 2000 Nov 1;351 Pt 3(Pt 3):669-76. Biochem J. 2000. PMID: 11042121 Free PMC article.
-
Src and Syk kinases: key regulators of phagocytic cell activation.Trends Immunol. 2005 Apr;26(4):208-14. doi: 10.1016/j.it.2005.02.002. Trends Immunol. 2005. PMID: 15797511 Review.
-
Integrin alpha(IIb)beta(3) signaling in platelet adhesion and aggregation.Curr Opin Cell Biol. 1999 Oct;11(5):597-601. doi: 10.1016/s0955-0674(99)00018-6. Curr Opin Cell Biol. 1999. PMID: 10508650 Review.
Cited by
-
Syk-dependent actin dynamics regulate endocytic trafficking and processing of antigens internalized through the B-cell receptor.Mol Biol Cell. 2007 Sep;18(9):3451-62. doi: 10.1091/mbc.e06-12-1114. Epub 2007 Jun 27. Mol Biol Cell. 2007. PMID: 17596518 Free PMC article.
-
Multiple sites on Streptococcus gordonii surface protein PadA bind to platelet GPIIbIIIa.Thromb Haemost. 2013 Dec;110(6):1278-1287. doi: 10.1160/TH13-07-0580. Epub 2013 Oct 17. Thromb Haemost. 2013. PMID: 24136582 Free PMC article.
-
Siglec-8 Signals Through a Non-Canonical Pathway to Cause Human Eosinophil Death In Vitro.Front Immunol. 2021 Oct 11;12:737988. doi: 10.3389/fimmu.2021.737988. eCollection 2021. Front Immunol. 2021. PMID: 34721399 Free PMC article.
-
The tyrosine phosphatase CD148 is an essential positive regulator of platelet activation and thrombosis.Blood. 2009 May 14;113(20):4942-54. doi: 10.1182/blood-2008-08-174318. Epub 2009 Feb 25. Blood. 2009. PMID: 19246339 Free PMC article.
-
The interplay between the proteolytic, invasive, and adhesive domains of invadopodia and their roles in cancer invasion.Cell Adh Migr. 2014;8(3):215-25. doi: 10.4161/cam.27842. Cell Adh Migr. 2014. PMID: 24714132 Free PMC article. Review.
References
-
- Aplin, A.E., A. Howe, S.K. Alahari, and R.L. Juliano. 1998. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol. Rev. 50:197–263. - PubMed
-
- Bazzoni, G., and M.E. Hemler. 1998. Are changes in integrin affinity and conformation overemphasized? Trends Biochem. Sci. 23:30–34. - PubMed
-
- Brdicka, T., D. Pavlistova, A. Leo, E. Bruyns, V. Korinek, P. Angelisova, J. Scherer, A. Shevchenko, I. Hilgert, J. Cerny, et al. 2000. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 191:1591–1604. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

