The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding
- PMID: 11937491
- PMCID: PMC186329
- DOI: 10.1101/gad.966402
The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding
Abstract
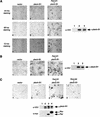
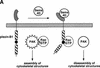
The small GTPase Rac has been implicated in growth cone guidance mediated by semaphorins and their receptors. Here we demonstrate that plexin-B1, a receptor for Semaphorin4D (Sema4D), and p21-activated kinase (PAK) can compete for the interaction with active Rac and plexin-B1 can inhibit Rac-induced PAK activation. We have also demonstrated that expression of active Rac enhances the ability of plexin-B1 to interact with Sema4D. Active Rac stimulates the localization of plexin-B1 to the cell surface. The enhancement in Sema4D binding depends on the ability of Rac to bind plexin-B1. These observations support a model where signaling between Rac and plexin-B1 is bidirectional; Rac modulates plexin-B1 activity and plexin-B1 modulates Rac function.
Figures






Similar articles
-
The semaphorin receptor plexin-B1 specifically interacts with active Rac in a ligand-dependent manner.Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12457-62. doi: 10.1073/pnas.220421797. Proc Natl Acad Sci U S A. 2000. PMID: 11035813 Free PMC article.
-
Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B.Cancer Res. 2004 Aug 1;64(15):5212-24. doi: 10.1158/0008-5472.CAN-04-0126. Cancer Res. 2004. PMID: 15289326
-
Plexin-B family members demonstrate non-redundant expression patterns in the developing mouse nervous system: an anatomical basis for morphogenetic effects of Sema4D during development.Eur J Neurosci. 2004 May;19(10):2622-32. doi: 10.1111/j.0953-816X.2004.03401.x. Eur J Neurosci. 2004. PMID: 15147296
-
Biological functions and signaling of a transmembrane semaphorin, CD100/Sema4D.Cell Mol Life Sci. 2004 Feb;61(3):292-300. doi: 10.1007/s00018-003-3257-7. Cell Mol Life Sci. 2004. PMID: 14770294 Free PMC article. Review.
-
Roles of Sema4D and Plexin-B1 in tumor progression.Mol Cancer. 2010 Sep 21;9:251. doi: 10.1186/1476-4598-9-251. Mol Cancer. 2010. PMID: 20858260 Free PMC article. Review.
Cited by
-
Structure and function of the intracellular region of the plexin-b1 transmembrane receptor.J Biol Chem. 2009 Dec 18;284(51):35962-72. doi: 10.1074/jbc.M109.056275. J Biol Chem. 2009. PMID: 19843518 Free PMC article.
-
Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning.Trends Cell Biol. 2009 Mar;19(3):99-110. doi: 10.1016/j.tcb.2009.01.001. Epub 2009 Feb 4. Trends Cell Biol. 2009. PMID: 19200729 Free PMC article. Review.
-
Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization.Sci Signal. 2012 Jan 17;5(207):ra6. doi: 10.1126/scisignal.2002636. Sci Signal. 2012. PMID: 22253263 Free PMC article.
-
Immunological functions of the neuropilins and plexins as receptors for semaphorins.Nat Rev Immunol. 2013 Nov;13(11):802-14. doi: 10.1038/nri3545. Nat Rev Immunol. 2013. PMID: 24319778 Review.
-
A Comprehensive Review of Dysregulated miRNAs Involved in Cervical Cancer.Curr Genomics. 2014 Aug;15(4):310-23. doi: 10.2174/1389202915666140528003249. Curr Genomics. 2014. PMID: 25132800 Free PMC article.
References
-
- Bentley D, O'Connor TP. Cytoskeletal events in growth cone steering. Curr Opin Neurobiol. 1994;4:43–48. - PubMed
-
- Culotti JG, Kolodkin AL. Functions of netrins and semaphorins in axon guidance. Curr Opin Neurobiol. 1996;6:81–88. - PubMed
-
- Driessens MH, Hu H, Nobes CD, Self A, Jordens I, Goodman CS, Hall A. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol. 2001;11:339–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
