Quaternary structure of coronavirus spikes in complex with carcinoembryonic antigen-related cell adhesion molecule cellular receptors
- PMID: 11912215
- PMCID: PMC8060896
- DOI: 10.1074/jbc.M201837200
Quaternary structure of coronavirus spikes in complex with carcinoembryonic antigen-related cell adhesion molecule cellular receptors
Abstract
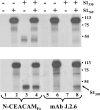
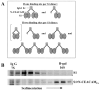
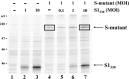
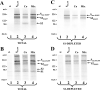
Oligomeric spike (S) glycoproteins extend from coronavirus membranes. These integral membrane proteins assemble within the endoplasmic reticulum of infected cells and are subsequently endoproteolyzed in the Golgi, generating noncovalently associated S1 and S2 fragments. Once on the surface of infected cells and virions, peripheral S1 fragments bind carcinoembryonic antigen-related cell adhesion molecule (CEACAM) receptors, and this triggers membrane fusion reactions mediated by integral membrane S2 fragments. We focused on the quaternary structure of S and its interaction with CEACAMs. We discovered that soluble S1 fragments were dimers and that CEACAM binding was entirely dependent on this quaternary structure. However, two differentially tagged CEACAMs could not co-precipitate with the S dimers, suggesting that binding sites were closely juxtaposed in the dimer (steric hindrance) or that a single CEACAM generated global conformational changes that precluded additional interactions (negative cooperativity). CEACAM binding did indeed alter S1 conformations, generating alternative disulfide linkages that were revealed on SDS gels. CEACAM binding also induced separation of S1 and S2. Differentially tagged S2 fragments that were free of S1 dimers were not co-precipitated, suggesting that S1 harbored the primary oligomerization determinants. We discuss the distinctions between the S.CEACAM interaction and other virus-receptor complexes involved in receptor-triggered entry.
Figures





Similar articles
-
Conformational changes in the spike glycoprotein of murine coronavirus are induced at 37 degrees C either by soluble murine CEACAM1 receptors or by pH 8.J Virol. 2003 Jan;77(2):830-40. doi: 10.1128/jvi.77.2.830-840.2003. J Virol. 2003. PMID: 12502799 Free PMC article.
-
Variations in disparate regions of the murine coronavirus spike protein impact the initiation of membrane fusion.J Virol. 2001 Mar;75(6):2792-802. doi: 10.1128/JVI.75.6.2792-2802.2001. J Virol. 2001. PMID: 11222703 Free PMC article.
-
Specific Binding to Differentially Expressed Human Carcinoembryonic Antigen-Related Cell Adhesion Molecules Determines the Outcome of Neisseria gonorrhoeae Infections along the Female Reproductive Tract.Infect Immun. 2018 Jul 23;86(8):e00092-18. doi: 10.1128/IAI.00092-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29760215 Free PMC article.
-
Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis.Cancer Metastasis Rev. 2013 Dec;32(3-4):643-71. doi: 10.1007/s10555-013-9444-6. Cancer Metastasis Rev. 2013. PMID: 23903773 Review.
-
CEACAMs: their role in physiology and pathophysiology.Curr Opin Cell Biol. 2006 Oct;18(5):565-71. doi: 10.1016/j.ceb.2006.08.008. Epub 2006 Aug 17. Curr Opin Cell Biol. 2006. PMID: 16919437 Free PMC article. Review.
Cited by
-
Conformational changes in the spike glycoprotein of murine coronavirus are induced at 37 degrees C either by soluble murine CEACAM1 receptors or by pH 8.J Virol. 2003 Jan;77(2):830-40. doi: 10.1128/jvi.77.2.830-840.2003. J Virol. 2003. PMID: 12502799 Free PMC article.
-
Entry of viruses through the epithelial barrier: pathogenic trickery.Nat Rev Mol Cell Biol. 2003 Jan;4(1):57-68. doi: 10.1038/nrm1005. Nat Rev Mol Cell Biol. 2003. PMID: 12511869 Free PMC article. Review.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
-
The avian coronavirus spike protein.Virus Res. 2014 Dec 19;194:37-48. doi: 10.1016/j.virusres.2014.10.009. Epub 2014 Oct 17. Virus Res. 2014. PMID: 25451062 Free PMC article. Review.
-
Aromatic amino acids in the juxtamembrane domain of severe acute respiratory syndrome coronavirus spike glycoprotein are important for receptor-dependent virus entry and cell-cell fusion.J Virol. 2008 Mar;82(6):2883-94. doi: 10.1128/JVI.01805-07. Epub 2008 Jan 16. J Virol. 2008. PMID: 18199653 Free PMC article.
References
-
- Hernandez L.D., Hoffman L.R., Wolfsberg T.G., White J.M. Annu. Rev. Cell Dev. Biol. 1986;12:627–661. - PubMed
-
- Wu L., Gerard N.P., Wyatt R., Choe H., Parolin C., Ruffing N., Borsetti A., Cardoso A.A., Desjardin E., Newman W., Gerard C., Sodroski J. Nature. 1996;384:179–183. - PubMed
-
- Mothes W., Boerger A.L., Narayan S., Cunningham J.M., Young J.A. Cell. 2000;103:679–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

