Role of adaptor complex AP-3 in targeting wild-type and mutated CD63 to lysosomes
- PMID: 11907283
- PMCID: PMC99620
- DOI: 10.1091/mbc.01-08-0409
Role of adaptor complex AP-3 in targeting wild-type and mutated CD63 to lysosomes
Abstract
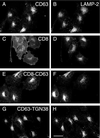
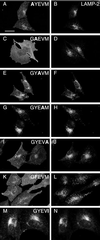
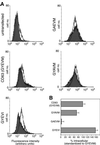
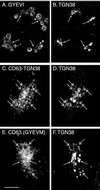
CD63 is a lysosomal membrane protein that belongs to the tetraspanin family. Its carboxyterminal cytoplasmic tail sequence contains the lysosomal targeting motif GYEVM. Strong, tyrosine-dependent interaction of the wild-type carboxyterminal tail of CD63 with the AP-3 adaptor subunit mu 3 was observed using a yeast two-hybrid system. The strength of interaction of mutated tail sequences with mu 3 correlated with the degree of lysosomal localization of similarly mutated human CD63 molecules in stably transfected normal rat kidney cells. Mutated CD63 containing the cytosolic tail sequence GYEVI, which interacted strongly with mu 3 but not at all with mu 2 in the yeast two-hybrid system, localized to lysosomes in transfected normal rat kidney and NIH-3T3 cells. In contrast, it localized to the cell surface in transfected cells of pearl and mocha mice, which have genetic defects in genes encoding subunits of AP-3, but to lysosomes in functionally rescued mocha cells expressing the delta subunit of AP-3. Thus, AP-3 is absolutely required for the delivery of this mutated CD63 to lysosomes. Using this AP-3-dependent mutant of CD63, we have shown that AP-3 functions in membrane traffic from the trans-Golgi network to lysosomes via an intracellular route that appears to bypass early endosomes.
Figures

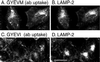
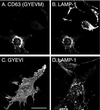
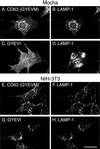
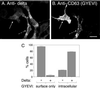
Similar articles
-
Differential use of two AP-3-mediated pathways by lysosomal membrane proteins.Traffic. 2004 Dec;5(12):946-62. doi: 10.1111/j.1600-0854.2004.00236.x. Traffic. 2004. PMID: 15522097
-
Lysosomal Targeting of Cystinosin Requires AP-3.Traffic. 2015 Jul;16(7):712-26. doi: 10.1111/tra.12277. Epub 2015 Mar 24. Traffic. 2015. PMID: 25753619
-
P-selectin and CD63 use different mechanisms for delivery to Weibel-Palade bodies.Traffic. 2006 Jun;7(6):647-62. doi: 10.1111/j.1600-0854.2006.00415.x. Traffic. 2006. PMID: 16683915
-
BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes.Mol Biol Cell. 2006 Sep;17(9):4027-38. doi: 10.1091/mbc.e06-05-0379. Epub 2006 Jul 12. Mol Biol Cell. 2006. PMID: 16837549 Free PMC article.
-
Trafficking and function of the tetraspanin CD63.Exp Cell Res. 2009 May 15;315(9):1584-92. doi: 10.1016/j.yexcr.2008.09.020. Epub 2008 Oct 7. Exp Cell Res. 2009. PMID: 18930046 Review.
Cited by
-
Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction.Elife. 2016 Aug 25;5:e19276. doi: 10.7554/eLife.19276. Elife. 2016. PMID: 27559612 Free PMC article.
-
Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets.Expert Rev Mol Med. 2010 Jan 18;12:e3. doi: 10.1017/S1462399409001355. Expert Rev Mol Med. 2010. PMID: 20078909 Free PMC article. Review.
-
Interaction of Rab31 and OCRL-1 in oligodendrocytes: its role in transport of mannose 6-phosphate receptors.J Neurosci Res. 2010 Feb 15;88(3):589-604. doi: 10.1002/jnr.22236. J Neurosci Res. 2010. PMID: 19795375 Free PMC article.
-
In Vitro Disease Modeling of Hermansky-Pudlak Syndrome Type 2 Using Human Induced Pluripotent Stem Cell-Derived Alveolar Organoids.Stem Cell Reports. 2019 Mar 5;12(3):431-440. doi: 10.1016/j.stemcr.2019.01.014. Epub 2019 Feb 14. Stem Cell Reports. 2019. PMID: 30773483 Free PMC article.
-
The tumour-associated antigen L6 (L6-Ag) is recruited to the tetraspanin-enriched microdomains: implication for tumour cell motility.J Cell Sci. 2008 Mar 1;121(Pt 5):685-94. doi: 10.1242/jcs.020347. Epub 2008 Feb 12. J Cell Sci. 2008. PMID: 18270265 Free PMC article.
References
-
- Akasaki K, Michihara A, Mibuka K, Fujiwara Y, Tsuji H. Biosynthetic transport of a major lysosomal membrane glycoprotein, lamp-1: Convergence of biosynthetic and endocytic pathways occurs at three distinctive points. Exp Cell Res. 1995;220:464–473. - PubMed
-
- Akasaki K, Michihara A, Fujiwara Y, Mibuka K, Tsuji H. Biosynthetic transport of a major lysosome-associated membrane glycoprotein 2, lamp-2: a significant fraction of newly synthesized lamp-2 is delivered to lysosomes by way of early endosomes. J Biochem. 1996;120:1088–1094. - PubMed
-
- Andrejewski N, Punnonen E-L, Guhde G, Tanaka Y, Lüllmann-Rauch R, Hartmann D, von Figura K, Saftig P. Normal lysosomal morphology and function in LAMP-1-deficient mice. J Biol Chem. 1999;274:12692–12701. - PubMed
-
- Banting G, Maile R, Roquemore EP. The steady state distribution of humTGN46 is not significantly altered in cells defective in clathrin-mediated endocytosis. J Cell Sci. 1998;111:3451–3458. - PubMed
-
- Barriocanal JG, Bonifacino JS, Yuan L, Sandoval IV. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986;261:16755–16763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

