Minute virus of mice NS1 interacts with the SMN protein, and they colocalize in novel nuclear bodies induced by parvovirus infection
- PMID: 11907229
- PMCID: PMC136105
- DOI: 10.1128/jvi.76.8.3892-3904.2002
Minute virus of mice NS1 interacts with the SMN protein, and they colocalize in novel nuclear bodies induced by parvovirus infection
Abstract
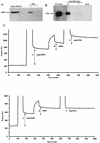
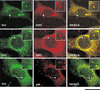
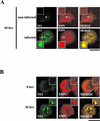
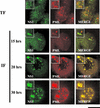
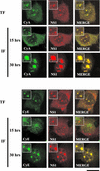
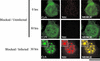
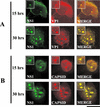
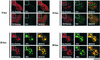
The human survival motor neuron (SMN) gene is the spinal muscular atrophy-determining gene, and a knockout of the murine Smn gene results in preembryonic lethality. Here we show that SMN can directly interact in vitro and in vivo with the large nonstructural protein NS1 of the autonomous parvovirus minute virus of mice (MVM), a protein essential for viral replication and a potent transcriptional activator. Typically, SMN localizes within nuclear Cajal bodies and diffusely in the cytoplasm. Following transient NS1expression, SMN and NS1 colocalize within Cajal bodies. At early time points following parvovirus infection, NS1 fails to colocalize with SMN within Cajal bodies; however, during the course of MVM infection, dramatic nuclear alterations occur. Formerly distinct nuclear bodies such as Cajal bodies, promyelocytic leukemia gene product (PML) oncogenic domains (PODs), speckles, and autonomous parvovirus-associated replication (APAR) bodies are seen aggregating at later points in infection. These newly formed large nuclear bodies (termed SMN-associated APAR bodies) are active sites of viral replication and viral capsid assembly. These results highlight the transient nature of nuclear bodies and their contents and identify a novel nuclear body formed during infection. Furthermore, simple transient expression of the viral nonstructural proteins is insufficient to induce this nuclear reorganization, suggesting that this event is induced specifically by a step in the viral infection process.
Figures


Similar articles
-
Minute virus of mice small non-structural protein NS2 localizes within, but is not required for the formation of, Smn-associated autonomous parvovirus-associated replication bodies.J Gen Virol. 2005 Apr;86(Pt 4):1009-1014. doi: 10.1099/vir.0.80564-0. J Gen Virol. 2005. PMID: 15784894
-
Minute virus of mice small nonstructural protein NS2 interacts and colocalizes with the Smn protein.J Virol. 2002 Jun;76(12):6364-9. doi: 10.1128/jvi.76.12.6364-6369.2002. J Virol. 2002. PMID: 12021369 Free PMC article.
-
Coilin forms the bridge between Cajal bodies and SMN, the spinal muscular atrophy protein.Genes Dev. 2001 Oct 15;15(20):2720-9. doi: 10.1101/gad.908401. Genes Dev. 2001. PMID: 11641277 Free PMC article.
-
Macromolecular complexes: SMN--the master assembler.Curr Biol. 2001 Oct 30;11(21):R862-4. doi: 10.1016/s0960-9822(01)00517-6. Curr Biol. 2001. PMID: 11696342 Review.
-
The SMN complex.Exp Cell Res. 2004 May 15;296(1):51-6. doi: 10.1016/j.yexcr.2004.03.022. Exp Cell Res. 2004. PMID: 15120993 Review.
Cited by
-
Replication initiator protein NS1 of the parvovirus minute virus of mice binds to modular divergent sites distributed throughout duplex viral DNA.J Virol. 2007 Dec;81(23):13015-27. doi: 10.1128/JVI.01703-07. Epub 2007 Sep 26. J Virol. 2007. PMID: 17898054 Free PMC article.
-
The NS2 proteins of parvovirus minute virus of mice are required for efficient nuclear egress of progeny virions in mouse cells.J Virol. 2002 Oct;76(20):10307-19. doi: 10.1128/jvi.76.20.10307-10319.2002. J Virol. 2002. PMID: 12239307 Free PMC article.
-
Effect of ATP binding and hydrolysis on dynamics of canine parvovirus NS1.J Virol. 2010 May;84(10):5391-403. doi: 10.1128/JVI.02221-09. Epub 2010 Mar 10. J Virol. 2010. PMID: 20219935 Free PMC article.
-
Mosquito densonucleosis virus non-structural protein NS2 is necessary for a productive infection.Virology. 2008 Apr 25;374(1):128-37. doi: 10.1016/j.virol.2007.11.035. Epub 2008 Jan 28. Virology. 2008. PMID: 18222517 Free PMC article.
-
Protoparvovirus Interactions with the Cellular DNA Damage Response.Viruses. 2017 Oct 31;9(11):323. doi: 10.3390/v9110323. Viruses. 2017. PMID: 29088070 Free PMC article. Review.
References
-
- Ahn, J. H., and G. S. Hayward. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 274:39-55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

