Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus
- PMID: 11907214
- PMCID: PMC136081
- DOI: 10.1128/jvi.76.8.3748-3755.2002
Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus
Abstract
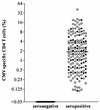
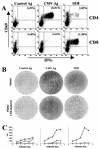
Replication of cytomegalovirus (CMV) is largely controlled by the cellular arm of the immune response. In this study the CMV-specific CD4 T-cell response was characterized in a cohort of apparently healthy individuals. In 11% of all individuals, extremely high frequencies, between 10 and 40%, were found. High-level frequencies of CMV-specific CD4 T cells persisted over several months and were not the result of an acute infection. Specific T cells were oligoclonal and were phenotypically and functionally characterized as mature effector cells, with both cytokine-secreting and proliferative potential. These high-level frequencies do not seem to compromise the immune response towards heterologous infections, and no signs of immunopathology were observed. Whereas a large temporary expansion of virus-specific T cells is well known to occur during acute infection, we now show that extremely high frequencies of virus-specific T cells may continuously exist in chronic CMV infection without overtly compromising the remaining protective immunity.
Figures




Similar articles
-
Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection.J Am Soc Nephrol. 2002 Oct;13(10):2577-84. doi: 10.1097/01.asn.0000030141.41726.52. J Am Soc Nephrol. 2002. PMID: 12239248
-
[Monitoring of cytomegalovirus-specific CD4+ and CD8+ T cell responses by cytokine flow cytometry in renal transplant recipients].Mikrobiyol Bul. 2016 Apr;50(2):224-35. Mikrobiyol Bul. 2016. PMID: 27175495 Turkish.
-
Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation.Transplantation. 2001 May 15;71(9):1287-94. doi: 10.1097/00007890-200105150-00018. Transplantation. 2001. PMID: 11397964
-
Analyzing T-cell responses to cytomegalovirus by cytokine flow cytometry.Hum Immunol. 2004 May;65(5):493-9. doi: 10.1016/j.humimm.2004.02.004. Hum Immunol. 2004. PMID: 15172449 Review.
-
Cytomegalovirus (CMV)-specific cellular immune responses.Hum Immunol. 2004 May;65(5):500-6. doi: 10.1016/j.humimm.2004.02.012. Hum Immunol. 2004. PMID: 15172450 Review.
Cited by
-
Evolving models of the immunopathogenesis of T cell-mediated drug allergy: The role of host, pathogens, and drug response.J Allergy Clin Immunol. 2015 Aug;136(2):219-34; quiz 235. doi: 10.1016/j.jaci.2015.05.050. J Allergy Clin Immunol. 2015. PMID: 26254049 Free PMC article. Review.
-
Inflammation in HIV and Its Impact on Atherosclerotic Cardiovascular Disease.Circ Res. 2024 May 24;134(11):1515-1545. doi: 10.1161/CIRCRESAHA.124.323891. Epub 2024 May 23. Circ Res. 2024. PMID: 38781301 Free PMC article. Review.
-
Human cytomegalovirus tropism for endothelial cells: not all endothelial cells are created equal.J Virol. 2007 Mar;81(5):2095-101. doi: 10.1128/JVI.01422-06. Epub 2006 Sep 6. J Virol. 2007. PMID: 16956936 Free PMC article. Review. No abstract available.
-
Primary response against cytomegalovirus during antiviral prophylaxis with valganciclovir, in solid organ transplant recipients.Transpl Int. 2011 Sep;24(9):920-31. doi: 10.1111/j.1432-2277.2011.01285.x. Epub 2011 Jun 14. Transpl Int. 2011. PMID: 21672050 Free PMC article.
-
The immune response to cytomegalovirus in allogeneic hematopoietic stem cell transplant recipients.Cell Mol Life Sci. 2015 Nov;72(21):4049-62. doi: 10.1007/s00018-015-1986-z. Epub 2015 Jul 15. Cell Mol Life Sci. 2015. PMID: 26174234 Free PMC article. Review.
References
-
- Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. - PMC - PubMed
-
- Callan, M. F., N. Steven, P. Krausa, J. D. Wilson, P. A. Moss, G. M. Gillespie, J. I. Bell, A. B. Rickinson, and A. J. McMichael. 1996. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat. Med. 2:906-911. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

