Solution structure of a Bcl-2 homolog from Kaposi sarcoma virus
- PMID: 11904405
- PMCID: PMC122540
- DOI: 10.1073/pnas.062525799
Solution structure of a Bcl-2 homolog from Kaposi sarcoma virus
Abstract
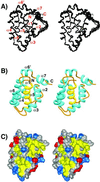
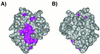
Kaposi sarcoma-associated herpes virus (KSHV) contains a gene that has functional and sequence homology to the apoptotic Bcl-2 family of proteins [Sarid, R., Sato, T., Bohenzky, R. A., Russo, J. J. & Chang, Y. (1997) Nat. Med. 3, 293-298]. The viral Bcl-2 protein promotes survival of infected cells and may contribute to the development of Kaposi sarcoma tumors [Boshoff, C. & Chang, Y. (2001) Annu. Rev. Med. 52, 453-470]. Here we describe the solution structure of the viral Bcl-2 homolog from KSHV. Comparison of the KSHV Bcl-2 structure to that of Bcl-2 and Bcl-x(L) shows that although the overall fold is the same, there are key differences in the lengths of the helices and loops. Binding studies on peptides derived from the Bcl-2 homology region 3 of proapoptotic family members indicate that the specificity of the viral protein is very different from what was previously observed for Bcl-x(L) and Bcl-2, suggesting that the viral protein has evolved to have a different mechanism of action than the host proteins.
Figures

Similar articles
-
A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak.Proc Natl Acad Sci U S A. 1997 Jan 21;94(2):690-4. doi: 10.1073/pnas.94.2.690. Proc Natl Acad Sci U S A. 1997. PMID: 9012846 Free PMC article.
-
Structural biology of the Bcl-2 family of proteins.Biochim Biophys Acta. 2004 Mar 1;1644(2-3):83-94. doi: 10.1016/j.bbamcr.2003.08.012. Biochim Biophys Acta. 2004. PMID: 14996493 Review.
-
Structural basis for the conserved binding mechanism of MDM2-inhibiting peptides and anti-apoptotic Bcl-2 family proteins.Biochem Biophys Res Commun. 2014 Feb 28;445(1):120-5. doi: 10.1016/j.bbrc.2014.01.130. Epub 2014 Feb 1. Biochem Biophys Res Commun. 2014. PMID: 24491548
-
Nuclear magnetic resonance study of protein-protein interactions involving apoptosis regulator Diva (Boo) and the BH3 domain of proapoptotic Bcl-2 members.J Mol Recognit. 2012 Dec;25(12):665-73. doi: 10.1002/jmr.2240. J Mol Recognit. 2012. PMID: 23192964
-
BH3-only proteins - evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death.J Cell Sci. 2002 Apr 15;115(Pt 8):1567-74. doi: 10.1242/jcs.115.8.1567. J Cell Sci. 2002. PMID: 11950875 Review.
Cited by
-
Structural studies of apoptosis and ion transport regulatory proteins in membranes.Magn Reson Chem. 2004 Feb;42(2):172-9. doi: 10.1002/mrc.1322. Magn Reson Chem. 2004. PMID: 14745797 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus and innate immunity.Future Virol. 2010 Mar 1;5(2):185-196. doi: 10.2217/fvl.10.5. Future Virol. 2010. PMID: 20414330 Free PMC article.
-
Viral product trafficking to mitochondria, mechanisms and roles in pathogenesis.Infect Disord Drug Targets. 2012 Feb;12(1):18-37. doi: 10.2174/187152612798994948. Infect Disord Drug Targets. 2012. PMID: 22034933 Free PMC article. Review.
-
Modulation of Immune System by Kaposi's Sarcoma-Associated Herpesvirus: Lessons from Viral Evasion Strategies.Front Microbiol. 2012 Mar 5;3:44. doi: 10.3389/fmicb.2012.00044. eCollection 2012. Front Microbiol. 2012. PMID: 22403573 Free PMC article.
-
The autophagy effector Beclin 1: a novel BH3-only protein.Oncogene. 2008 Dec;27 Suppl 1(Suppl 1):S137-48. doi: 10.1038/onc.2009.51. Oncogene. 2008. PMID: 19641499 Free PMC article. Review.
References
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

