Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors
- PMID: 11891196
- PMCID: PMC1867175
- DOI: 10.1016/S0002-9440(10)64920-6
Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors
Abstract
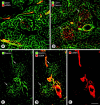
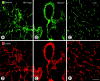
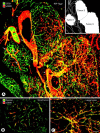
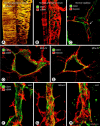
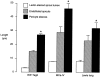
Endothelial cells of tumor vessels have well-documented alterations, but it is less clear whether pericytes on these vessels are abnormal or even absent. Here we report that alpha-smooth muscle actin (alpha-SMA) and desmin-immunoreactive pericytes were present on >97% of blood vessels viewed by confocal microscopy in 100-microm-thick sections of three different spontaneous or implanted tumors in mice. However, the cells had multiple abnormalities. Unlike pericytes on capillaries in normal pancreatic islets, which had desmin but not alpha-SMA immunoreactivity, pericytes on capillary-size vessels in insulinomas in RIP-Tag2 transgenic mice expressed both desmin and alpha-SMA. Furthermore, pericytes in RIP-Tag2 tumors, as well as those in MCa-IV breast carcinomas and Lewis lung carcinomas, had an abnormally loose association with endothelial cells and extended cytoplasmic processes deep into the tumor tissue. alpha-SMA-positive pericytes also covered 73% of endothelial sprouts in RIP-Tag2 tumors and 92% of sprouts in the other tumors. Indeed, pericyte sleeves were significantly longer than the CD31-immunoreactive endothelial cell sprouts themselves in all three types of tumors. All three tumors also contained alpha-SMA-positive myofibroblasts that resembled pericytes but were not associated with blood vessels. We conclude that pericytes are present on most tumor vessels but have multiple abnormalities, including altered expression of marker proteins. In contrast to some previous studies, the almost ubiquitous presence of pericytes on tumor vessels found in the present study may be attributed to our use of both desmin and alpha-SMA as markers and 100-microm-thick tissue sections. The association of pericytes with endothelial sprouts raises the possibility of an involvement in sprout growth or retraction in tumors.
Figures
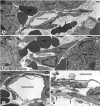
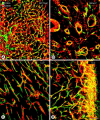
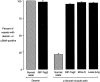
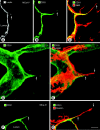

Similar articles
-
Rapid vascular regrowth in tumors after reversal of VEGF inhibition.J Clin Invest. 2006 Oct;116(10):2610-21. doi: 10.1172/JCI24612. J Clin Invest. 2006. PMID: 17016557 Free PMC article.
-
Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102.Cancer Res. 2007 Aug 1;67(15):7358-67. doi: 10.1158/0008-5472.CAN-07-0293. Cancer Res. 2007. PMID: 17671206 Free PMC article.
-
Angiogenesis with pericyte abnormalities in a transgenic model of prostate carcinoma.Cancer. 2005 Nov 15;104(10):2104-15. doi: 10.1002/cncr.21436. Cancer. 2005. PMID: 16208706
-
Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche.Histol Histopathol. 2009 Jul;24(7):909-69. doi: 10.14670/HH-24.909. Histol Histopathol. 2009. PMID: 19475537 Review.
-
Angiogenesis in wound healing and tumor metastasis.Behring Inst Mitt. 1993 Aug;(92):258-72. Behring Inst Mitt. 1993. PMID: 7504453 Review.
Cited by
-
Biglycan is a specific marker and an autocrine angiogenic factor of tumour endothelial cells.Br J Cancer. 2012 Mar 13;106(6):1214-23. doi: 10.1038/bjc.2012.59. Epub 2012 Feb 28. Br J Cancer. 2012. PMID: 22374465 Free PMC article.
-
Activation of alternate prosurvival pathways accounts for acquired sunitinib resistance in U87MG glioma xenografts.J Pharmacol Exp Ther. 2012 Nov;343(2):509-19. doi: 10.1124/jpet.112.196097. Epub 2012 Aug 6. J Pharmacol Exp Ther. 2012. PMID: 22869928 Free PMC article.
-
Endogenous brain pericytes are widely activated and contribute to mouse glioma microvasculature.PLoS One. 2015 Apr 13;10(4):e0123553. doi: 10.1371/journal.pone.0123553. eCollection 2015. PLoS One. 2015. PMID: 25875288 Free PMC article.
-
Molecular basis for pericyte-induced capillary tube network assembly and maturation.Front Cell Dev Biol. 2022 Aug 22;10:943533. doi: 10.3389/fcell.2022.943533. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36072343 Free PMC article. Review.
-
Matrix metalloproteinases: changing roles in tumor progression and metastasis.Am J Pathol. 2012 Dec;181(6):1895-9. doi: 10.1016/j.ajpath.2012.08.044. Epub 2012 Oct 12. Am J Pathol. 2012. PMID: 23063657 Free PMC article. Review.
References
-
- Jain RK: The next frontier of molecular medicine: delivery of therapeutics. Nat Med 1998, 4:655-657 - PubMed
-
- Arap W, Pasqualini R, Ruoslahti E: Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279:377-380 - PubMed
-
- Pasqualini R: Vascular targeting with phage peptide libraries. Q J Nucl Med 1999, 43:159-162 - PubMed
-
- Folkman J: Tumor angiogenesis. Adv Cancer Res 1985, 43:175-203 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

