The TRAP/Mediator coactivator complex interacts directly with estrogen receptors alpha and beta through the TRAP220 subunit and directly enhances estrogen receptor function in vitro
- PMID: 11867769
- PMCID: PMC122401
- DOI: 10.1073/pnas.261715899
The TRAP/Mediator coactivator complex interacts directly with estrogen receptors alpha and beta through the TRAP220 subunit and directly enhances estrogen receptor function in vitro
Abstract
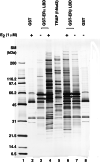
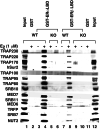
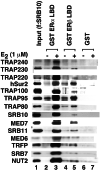
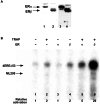
Target gene activation by nuclear hormone receptors, including estrogen receptors (ERs), is thought to be mediated by a variety of interacting cofactors. Here we identify a number of nuclear extract-derived proteins that interact with immobilized ER ligand binding domains in a 17beta-estradiol-dependent manner. The most prominent of these are components of the thyroid hormone receptor-associated protein (TRAP)/Mediator coactivator complex, which interacts with ERalpha and ERbeta in both unfractionated nuclear extracts and purified form. Studies with extracts from TRAP220(-/-) fibroblasts reveal that these interactions depend on TRAP220, a TRAP/Mediator subunit previously shown to interact with ER and other nuclear receptors in a ligand-dependent manner. The physiological relevance of the in vitro interaction is documented further by the isolation of an ERalpha-TRAP/Mediator complex from cultured cells expressing an epitope-tagged ERalpha. Finally, the complete TRAP/Mediator complex is shown to enhance ER function directly in a highly purified cell-free transcription system. These studies firmly establish a direct role for TRAP/Mediator, through TRAP220, in ER function.
Figures



Similar articles
-
Transcription coactivator TRAP220 is required for PPAR gamma 2-stimulated adipogenesis.Nature. 2002 May 30;417(6888):563-7. doi: 10.1038/417563a. Nature. 2002. PMID: 12037571
-
p300 mediates functional synergism between AF-1 and AF-2 of estrogen receptor alpha and beta by interacting directly with the N-terminal A/B domains.J Biol Chem. 2000 May 26;275(21):15645-51. doi: 10.1074/jbc.M000042200. J Biol Chem. 2000. PMID: 10747867
-
The TRAP220 component of a thyroid hormone receptor- associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion.Proc Natl Acad Sci U S A. 1998 Jul 7;95(14):7939-44. doi: 10.1073/pnas.95.14.7939. Proc Natl Acad Sci U S A. 1998. PMID: 9653119 Free PMC article.
-
The TRAP/SMCC/Mediator complex and thyroid hormone receptor function.Trends Endocrinol Metab. 2001 Apr;12(3):127-34. doi: 10.1016/s1043-2760(00)00355-6. Trends Endocrinol Metab. 2001. PMID: 11306338 Review.
-
Role of the mediator complex in nuclear hormone receptor signaling.Rev Physiol Biochem Pharmacol. 2006;156:23-43. doi: 10.1007/s10254-005-0002-0. Rev Physiol Biochem Pharmacol. 2006. PMID: 16634145 Review.
Cited by
-
Transcriptional complexes engaged by apo-estrogen receptor-alpha isoforms have divergent outcomes.EMBO J. 2004 Sep 15;23(18):3653-66. doi: 10.1038/sj.emboj.7600377. Epub 2004 Sep 2. EMBO J. 2004. PMID: 15343269 Free PMC article.
-
Mediator-dependent nuclear receptor function.Semin Cell Dev Biol. 2011 Sep;22(7):749-58. doi: 10.1016/j.semcdb.2011.07.026. Epub 2011 Aug 10. Semin Cell Dev Biol. 2011. PMID: 21854863 Free PMC article. Review.
-
Analysis of differential expression of Mediator subunit genes in Arabidopsis.Plant Signal Behav. 2012 Dec;7(12):1676-86. doi: 10.4161/psb.22438. Epub 2012 Oct 16. Plant Signal Behav. 2012. PMID: 23072992 Free PMC article.
-
Proteomic analysis of coregulators bound to ERα on DNA and nucleosomes reveals coregulator dynamics.Mol Cell. 2013 Jul 25;51(2):185-99. doi: 10.1016/j.molcel.2013.06.007. Epub 2013 Jul 11. Mol Cell. 2013. PMID: 23850489 Free PMC article.
-
Chromatin exposes intrinsic differences in the transcriptional activities of estrogen receptors alpha and beta.EMBO J. 2003 Feb 3;22(3):600-11. doi: 10.1093/emboj/cdg037. EMBO J. 2003. PMID: 12554660 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

