Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury
- PMID: 11867727
- PMCID: PMC122504
- DOI: 10.1073/pnas.052308899
Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury
Abstract
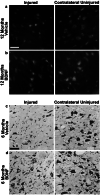
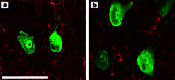
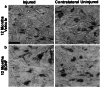
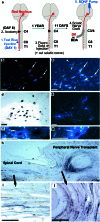
Scientific interest to find a treatment for spinal cord injuries has led to the development of numerous experimental strategies to promote axonal regeneration across the spinal cord injury site. Although these strategies have been developed in acute injury paradigms and hold promise for individuals with spinal cord injuries in the future, little is known about their applicability for the vast majority of paralyzed individuals whose injury occurred long ago and who are considered to have a chronic injury. Some studies have shown that the effectiveness of these approaches diminishes dramatically within weeks after injury. Here we investigated the regenerative capacity of rat rubrospinal neurons whose axons were cut in the cervical spinal cord 1 year before. Contrary to earlier reports, we found that rubrospinal neurons do not die after axotomy but, rather, they undergo massive atrophy that can be reversed by applying brain-derived neurotrophic factor to the cell bodies in the midbrain. This administration of neurotrophic factor to the cell body resulted in increased expression of growth-associated protein-43 and Talpha1 tubulin, genes thought to be related to axonal regeneration. This treatment promoted the regeneration of these chronically injured rubrospinal axons into peripheral nerve transplants engrafted at the spinal cord injury site. This outcome is a demonstration of the regenerative capacity of spinal cord projection neurons a full year after axotomy.
Figures


Similar articles
-
Brain-derived neurotrophic factor gene transfer with adeno-associated viral and lentiviral vectors prevents rubrospinal neuronal atrophy and stimulates regeneration-associated gene expression after acute cervical spinal cord injury.Spine (Phila Pa 1976). 2007 May 15;32(11):1164-73. doi: 10.1097/BRS.0b013e318053ec35. Spine (Phila Pa 1976). 2007. PMID: 17495772
-
Rubrospinal neurons fail to respond to brain-derived neurotrophic factor applied to the spinal cord injury site 2 months after cervical axotomy.Exp Neurol. 2004 Sep;189(1):45-57. doi: 10.1016/j.expneurol.2004.05.034. Exp Neurol. 2004. PMID: 15296835
-
Treatment of chronically injured spinal cord with neurotrophic factors stimulates betaII-tubulin and GAP-43 expression in rubrospinal tract neurons.J Neurosci Res. 2003 Nov 15;74(4):502-11. doi: 10.1002/jnr.10787. J Neurosci Res. 2003. PMID: 14598294
-
Promoting axonal regeneration in the central nervous system by enhancing the cell body response to axotomy.J Neurosci Res. 2002 Apr 1;68(1):1-6. doi: 10.1002/jnr.10176. J Neurosci Res. 2002. PMID: 11933043 Review.
-
Strategies to promote regeneration and recovery in the injured spinal cord.Neurosurg Clin N Am. 1990 Jul;1(3):751-9. Neurosurg Clin N Am. 1990. PMID: 2136167 Review.
Cited by
-
Spinal cord injury I: A synopsis of the basic science.Can Vet J. 2010 May;51(5):485-92. Can Vet J. 2010. PMID: 20676289 Free PMC article. Review.
-
Identification of regeneration-associated genes after central and peripheral nerve injury in the adult rat.BMC Neurosci. 2003 May 19;4:8. doi: 10.1186/1471-2202-4-8. BMC Neurosci. 2003. PMID: 12756057 Free PMC article.
-
The potential for cellular therapy combined with growth factors in spinal cord injury.Stem Cells Int. 2012;2012:826754. doi: 10.1155/2012/826754. Epub 2012 Oct 3. Stem Cells Int. 2012. PMID: 23091499 Free PMC article.
-
Transplantation of Neural Precursor Cells Attenuates Chronic Immune Environment in Cervical Spinal Cord Injury.Front Neurol. 2018 Jun 8;9:428. doi: 10.3389/fneur.2018.00428. eCollection 2018. Front Neurol. 2018. PMID: 29951030 Free PMC article.
-
A gene network perspective on axonal regeneration.Front Mol Neurosci. 2011 Nov 22;4:46. doi: 10.3389/fnmol.2011.00046. eCollection 2011. Front Mol Neurosci. 2011. PMID: 22125511 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

