Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt's lymphoma
- PMID: 11867523
- PMCID: PMC125896
- DOI: 10.1093/emboj/21.5.954
Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt's lymphoma
Abstract
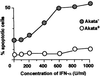
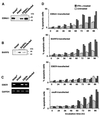
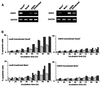
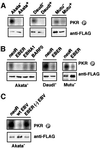
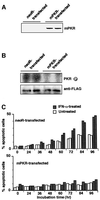
We investigated whether Epstein--Barr virus (EBV) infection could counteract the antitumor effect of interferon (IFN)-alpha. EBV-negative subclones isolated from EBV-positive Burkitt's lymphoma (BL) cell lines Akata, Daudi and Mutu were found to fall into apoptosis after IFN-alpha treatment. On the other hand, EBV-positive counterparts exhibited striking resistance against IFN-alpha-induced apoptosis. Transfection of an individual EBV latent gene into EBV-negative BL cells revealed that EBV-encoded poly(A)(-) RNAs (EBERs) were responsible for IFN resistance. EBERs bound double-stranded (ds) RNA-activated protein kinase (PKR), a key mediator of the antiviral effect of IFN-alpha, and inhibited its phosphorylation. Transfection of dominant-negative PKR, which was catalytically inactive and could block phosphorylation of endogenous PKR, made EBV-negative BL cells resistant to IFN-alpha-induced apoptosis. Furthermore, EBERs did not bind mutant PKR, which was catalytically active but lacked dsRNA-binding activity, nor did they inhibit its phosphorylation. These results indicate that EBERs confer resistance to IFN-alpha-induced apoptosis via binding to PKR and inhibition of its phosphorylation. This is the first report that the virus counteracts IFN-induced apoptosis in virus-associated tumors.
Figures









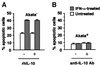
Similar articles
-
Protection from interferon-induced apoptosis by Epstein-Barr virus small RNAs is not mediated by inhibition of PKR.J Virol. 2005 Dec;79(23):14562-9. doi: 10.1128/JVI.79.23.14562-14569.2005. J Virol. 2005. PMID: 16282456 Free PMC article.
-
Epstein-Barr virus-encoded poly(A)(-) RNA supports Burkitt's lymphoma growth through interleukin-10 induction.EMBO J. 2000 Dec 15;19(24):6742-50. doi: 10.1093/emboj/19.24.6742. EMBO J. 2000. PMID: 11118209 Free PMC article.
-
The role of Epstein-Barr virus-encoded small RNAs (EBERs) in oncogenesis.Rev Med Virol. 2002 Sep-Oct;12(5):321-6. doi: 10.1002/rmv.363. Rev Med Virol. 2002. PMID: 12211044 Review.
-
Sensitivity of an epstein-barr virus-positive tumor line, Daudi, to alpha interferon correlates with expression of a GC-rich viral transcript.Mol Cell Biol. 1999 Nov;19(11):7305-13. doi: 10.1128/MCB.19.11.7305. Mol Cell Biol. 1999. PMID: 10523619 Free PMC article.
-
Modulation of innate immunity system by Epstein-Barr virus-encoded non-coding RNA and oncogenesis.Cancer Sci. 2010 Jan;101(1):29-35. doi: 10.1111/j.1349-7006.2009.01377.x. Epub 2009 Sep 29. Cancer Sci. 2010. PMID: 19886912 Free PMC article. Review.
Cited by
-
Expression of LINC00312, a long intergenic non-coding RNA, is negatively correlated with tumor size but positively correlated with lymph node metastasis in nasopharyngeal carcinoma.J Mol Histol. 2013 Oct;44(5):545-54. doi: 10.1007/s10735-013-9503-x. Epub 2013 Mar 26. J Mol Histol. 2013. PMID: 23529758
-
Prognostic role of ARID1A negative expression in gastric cancer.Sci Rep. 2019 May 1;9(1):6769. doi: 10.1038/s41598-019-43293-5. Sci Rep. 2019. PMID: 31043675 Free PMC article.
-
Adenovirus virus-associated RNAs induce type I interferon expression through a RIG-I-mediated pathway.J Virol. 2011 Apr;85(8):4035-40. doi: 10.1128/JVI.02160-10. Epub 2011 Jan 19. J Virol. 2011. PMID: 21248047 Free PMC article.
-
Current Insights into the Maturation of Epstein-Barr Virus Particles.Microorganisms. 2024 Apr 17;12(4):806. doi: 10.3390/microorganisms12040806. Microorganisms. 2024. PMID: 38674750 Free PMC article. Review.
-
Epstein-Barr virus infection and nasopharyngeal carcinoma.Philos Trans R Soc Lond B Biol Sci. 2017 Oct 19;372(1732):20160270. doi: 10.1098/rstb.2016.0270. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28893937 Free PMC article. Review.
References
-
- Alas S., Emmanouilides,C. and Bonavida,B. (2001) Inhibition of interleukin 10 by rituximab results in down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin’s lymphoma to apoptosis. Clin. Cancer Res., 7, 709–723. - PubMed
-
- Baer R. et al. (1984) DNA sequence and expression of the B95–8 Epstein–Barr virus genome. Nature, 310, 207–211. - PubMed
-
- Barber G.N. (2000) The interferons and cell death: guardians of the cell or accomplices of apoptosis. Semin. Cancer Biol., 10, 103–111. - PubMed
-
- Borden E.C., Lindner,D., Dreicer,R., Hussein,M. and Peereboom,D. (2000) Second-generation interferons for cancer: clinical targets. Semin. Cancer Biol., 10, 125–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

