Control of DNA replication and chromosome ploidy by geminin and cyclin A
- PMID: 11865064
- PMCID: PMC135598
- DOI: 10.1128/MCB.22.6.1868-1880.2002
Control of DNA replication and chromosome ploidy by geminin and cyclin A
Abstract
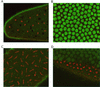
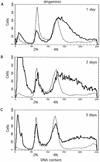
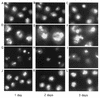
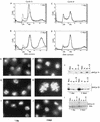
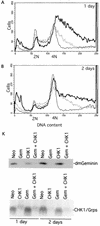
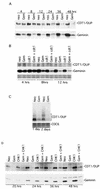
Alteration of the control of DNA replication and mitosis is considered to be a major cause of genome instability. To investigate the mechanism that controls DNA replication and genome stability, we used the RNA silencing-interference technique (RNAi) to eliminate the Drosophila geminin homologue from Schneider D2 (SD2) cells. Silencing of geminin by RNAi in SD2 cells leads to the cessation of mitosis and asynchronous overreplication of the genome, with cells containing single giant nuclei and partial ploidy between 4N and 8N DNA content. The effect of geminin deficiency is completely suppressed by cosilencing of Double parked (Dup), the Drosophila homologue of Cdt1, a replication factor to which geminin binds. The geminin deficiency-induced phenotype is also partially suppressed by coablation of Chk1/Grapes, indicating the involvement of Chk1/Grapes in the checkpoint control in response to overreplication. We found that the silencing of cyclin A, but not of cyclin B, also promotes the formation of a giant nucleus and overreplication. However, in contrast to the effect of geminin knockout, cyclin A deficiency leads to the complete duplication of the genome from 4N to 8N. We observed that the silencing of geminin causes rapid downregulation of Cdt1/Dup, which may contribute to the observed partial overreplication in geminin-deficient cells. Analysis of cyclin A and geminin double knockout suggests that the effect of cyclin A deficiency is dominant over that of geminin deficiency for cell cycle arrest and overreplication. Together, our studies indicate that both cyclin A and geminin are required for the suppression of overreplication and for genome stability in Drosophila cells.
Figures








Similar articles
-
Rereplication by depletion of geminin is seen regardless of p53 status and activates a G2/M checkpoint.Mol Cell Biol. 2004 Aug;24(16):7140-50. doi: 10.1128/MCB.24.16.7140-7150.2004. Mol Cell Biol. 2004. PMID: 15282313 Free PMC article.
-
Preferential re-replication of Drosophila heterochromatin in the absence of geminin.PLoS Genet. 2010 Sep 9;6(9):e1001112. doi: 10.1371/journal.pgen.1001112. PLoS Genet. 2010. PMID: 20838463 Free PMC article.
-
Levels of the origin-binding protein Double parked and its inhibitor Geminin increase in response to replication stress.J Cell Sci. 2005 Sep 15;118(Pt 18):4207-17. doi: 10.1242/jcs.02534. Epub 2005 Sep 1. J Cell Sci. 2005. PMID: 16141238
-
Cdt1 and geminin: role during cell cycle progression and DNA damage in higher eukaryotes.Front Biosci. 2007 Jan 1;12:1629-41. doi: 10.2741/2175. Front Biosci. 2007. PMID: 17127409 Review.
-
Geminin-Cdt1 balance is critical for genetic stability.Mutat Res. 2005 Jan 6;569(1-2):111-21. doi: 10.1016/j.mrfmmm.2004.05.026. Mutat Res. 2005. PMID: 15603756 Review.
Cited by
-
An ATR- and BRCA1-mediated Fanconi anemia pathway is required for activating the G2/M checkpoint and DNA damage repair upon rereplication.Mol Cell Biol. 2006 Jun;26(12):4601-11. doi: 10.1128/MCB.02141-05. Mol Cell Biol. 2006. PMID: 16738325 Free PMC article.
-
Regulation of CDC6, geminin, and CDT1 in human cells that undergo polyploidization.Mol Biol Cell. 2002 Nov;13(11):3989-4000. doi: 10.1091/mbc.e02-04-0217. Mol Biol Cell. 2002. PMID: 12429841 Free PMC article.
-
Cdt1 and Cdc6 are destabilized by rereplication-induced DNA damage.J Biol Chem. 2008 Sep 12;283(37):25356-25363. doi: 10.1074/jbc.M802667200. Epub 2008 Jul 10. J Biol Chem. 2008. PMID: 18617514 Free PMC article.
-
Induction of endocycles represses apoptosis independently of differentiation and predisposes cells to genome instability.Development. 2014 Jan;141(1):112-23. doi: 10.1242/dev.098871. Epub 2013 Nov 27. Development. 2014. PMID: 24284207 Free PMC article.
-
Prevention of DNA re-replication in eukaryotic cells.J Mol Cell Biol. 2011 Feb;3(1):13-22. doi: 10.1093/jmcb/mjq052. J Mol Cell Biol. 2011. PMID: 21278447 Free PMC article. Review.
References
-
- Blasina, A., I. V. de Weyer, M. C. Laus, W. H. Luyten, A. E. Parker, and C. H. McGowan. 1999. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr. Biol. 9:1-10. - PubMed
-
- Calzada, A., M. Sanchez, E. Sanchez, and A. Bueno. 2000. The stability of the Cdc6 protein is regulated by cyclin-dependent kinase/cyclin B complexes in Saccharomyces cerevisiae. J. Biol. Chem. 275:9734-9741. - PubMed
-
- Chaturvedi, P., W. K. Eng, Y. Zhu, M. R. Mattern, R. Mishra, M. R. Hurle, X. Zhang, R. S. Annan, Q. Lu, L. F. Faucette, G. F. Scott, X. Li, S. A. Carr, R. K. Johnson, J. D. Winkler, and B. B. Zhou. 1999. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene 18:4047-4054. - PubMed
-
- Diffley, J. F. 1996. Once and only once upon a time: specifying and regulating origins of DNA replication in eukaryotic cells. Genes Dev. 10:2819-2830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
