RNAi in Dictyostelium: the role of RNA-directed RNA polymerases and double-stranded RNase
- PMID: 11854403
- PMCID: PMC65640
- DOI: 10.1091/mbc.01-04-0211
RNAi in Dictyostelium: the role of RNA-directed RNA polymerases and double-stranded RNase
Abstract
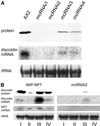
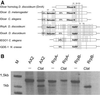
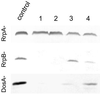
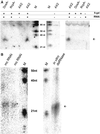
We show that in Dictyostelium discoideum an endogenous gene as well as a transgene can be silenced by introduction of a gene construct that is transcribed into a hairpin RNA. Gene silencing was accompanied by the appearance of sequence-specific RNA about 23mers and seemed to have a limited capacity. The three Dictyostelium homologues of the RNA-directed RNA polymerase (RrpA, RrpB, and DosA) all contain an N-terminal helicase domain homologous to the one in the dicer nuclease, suggesting exon shuffling between RNA-directed RNA polymerase and the dicer homologue. Only the knock-out of rrpA resulted in a loss of the hairpin RNA effect and simultaneously in a loss of detectable about 23mers. However, about 23mers were still generated by the Dictyostelium dsRNase in vitro with extracts from rrpA(-), rrpB(-), and DosA(-) cells. Both RrpA and a target gene were required for production of detectable amounts of about 23mers, suggesting that target sequences are involved in about 23mer amplification.
Figures

Similar articles
-
The 5' spreading of small RNAs in Dictyostelium discoideum depends on the RNA-dependent RNA polymerase RrpC and on the dicer-related nuclease DrnB.PLoS One. 2013 May 20;8(5):e64804. doi: 10.1371/journal.pone.0064804. Print 2013. PLoS One. 2013. PMID: 23724097 Free PMC article.
-
Use of a transactive regulatory mutant of Dictyostelium discoideum in a eucaryotic expression system.Nucleic Acids Res. 1993 Mar 25;21(6):1397-401. doi: 10.1093/nar/21.6.1397. Nucleic Acids Res. 1993. PMID: 8464730 Free PMC article.
-
Absence of complementation in non-allelic mutants of Dictyostelium discoideum with defects in the transition from growth to development.Mol Genet Genomics. 2001 Nov;266(3):396-405. doi: 10.1007/s004380100524. Mol Genet Genomics. 2001. PMID: 11713669
-
Expression of recombinant glycoproteins in the simple eukaryote Dictyostelium discoideum.Biotechnol Genet Eng Rev. 1997;14:1-35. doi: 10.1080/02648725.1997.10647937. Biotechnol Genet Eng Rev. 1997. PMID: 9188148 Review. No abstract available.
-
On the origin and functions of RNA-mediated silencing: from protists to man.Curr Genet. 2006 Aug;50(2):81-99. doi: 10.1007/s00294-006-0078-x. Epub 2006 May 12. Curr Genet. 2006. PMID: 16691418 Free PMC article. Review.
Cited by
-
RNA-dependent RNA polymerase is an essential component of a self-enforcing loop coupling heterochromatin assembly to siRNA production.Proc Natl Acad Sci U S A. 2005 Jan 4;102(1):152-7. doi: 10.1073/pnas.0407641102. Epub 2004 Dec 22. Proc Natl Acad Sci U S A. 2005. PMID: 15615848 Free PMC article.
-
RNA-mediated gene silencing in the cereal fungal pathogen Cochliobolus sativus.Mol Plant Pathol. 2011 Apr;12(3):289-98. doi: 10.1111/j.1364-3703.2010.00666.x. Epub 2010 Oct 1. Mol Plant Pathol. 2011. PMID: 21356000 Free PMC article.
-
Diverse Evolutionary Trajectories for Small RNA Biogenesis Genes in the Oomycete Genus Phytophthora.Front Plant Sci. 2016 Mar 15;7:284. doi: 10.3389/fpls.2016.00284. eCollection 2016. Front Plant Sci. 2016. PMID: 27014308 Free PMC article.
-
Effects of length and location on the cellular response to double-stranded RNA.Microbiol Mol Biol Rev. 2004 Sep;68(3):432-52, table of contents. doi: 10.1128/MMBR.68.3.432-452.2004. Microbiol Mol Biol Rev. 2004. PMID: 15353564 Free PMC article. Review.
-
A natural variant of a host RNA-dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana.Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):6297-302. doi: 10.1073/pnas.0304346101. Epub 2004 Apr 12. Proc Natl Acad Sci U S A. 2004. PMID: 15079073 Free PMC article.
References
-
- Bass BL. Double-stranded RNA as a template for gene silencing. Cell. 2000;101:235–238. - PubMed
-
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. - PubMed
-
- Catalanotto C, Azzalin G, Macino G, Cogoni C. Gene silencing in worms, and fungi. Nature. 2000;404:245. - PubMed
-
- Cogoni C, Macino G. Gene silencing in Neurospora crassarequires a protein homologous to RNA-directed RNA polymerase. Nature. 1999;399:166–169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

