Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance
- PMID: 11854356
- PMCID: PMC2193619
- DOI: 10.1084/jem.20010032
Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance
Abstract
Using a tumor model of spontaneously arising insulinomas expressing a defined tumor-associated antigen, we investigated whether tumor growth promotes cross-presentation and tolerance of tumor-specific T cells. We found that an advanced tumor burden enhanced cross-presentation of tumor-associated antigens to high avidity tumor-specific T cells, inducing T cell proliferation and limited effector function in vivo. However, contrary to other models, tumor-specific T cells were not tolerized despite a high tumor burden. In fact, in tumor-bearing mice, persistence and responsiveness of adoptively transferred tumor-specific T cells were enhanced. Accordingly, a potent T cell-mediated antitumor response could be elicited by intravenous administration of tumor-derived peptide and agonistic anti-CD40 antibody or viral immunization and reimmunization. Thus, in this model, tumor growth promotes activation of high avidity tumor-specific T cells instead of tolerance. Therefore, the host remains responsive to T cell immunotherapy.
Figures


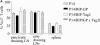





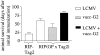
Similar articles
-
The fate of low affinity tumor-specific CD8+ T cells in tumor-bearing mice.J Immunol. 2005 Mar 1;174(5):2563-72. doi: 10.4049/jimmunol.174.5.2563. J Immunol. 2005. PMID: 15728462
-
Self antigens expressed by solid tumors Do not efficiently stimulate naive or activated T cells: implications for immunotherapy.J Exp Med. 1997 Aug 29;186(5):645-53. doi: 10.1084/jem.186.5.645. J Exp Med. 1997. PMID: 9271580 Free PMC article.
-
Cutting edge: tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation.J Immunol. 2004 Nov 15;173(10):5923-8. doi: 10.4049/jimmunol.173.10.5923. J Immunol. 2004. PMID: 15528325
-
Cross-presentation of tumour antigens: evaluation of threshold, duration, distribution and regulation.Immunol Cell Biol. 1999 Dec;77(6):552-8. doi: 10.1046/j.1440-1711.1999.00876.x. Immunol Cell Biol. 1999. PMID: 10571677 Review.
-
[Antitumor immunity and cellular cancer therapies].Med Sci (Paris). 2003 Jan;19(1):43-53. doi: 10.1051/medsci/200319143. Med Sci (Paris). 2003. PMID: 12836191 Review. French.
Cited by
-
Revisiting T Cell Tolerance as a Checkpoint Target for Cancer Immunotherapy.Front Immunol. 2020 Sep 23;11:589641. doi: 10.3389/fimmu.2020.589641. eCollection 2020. Front Immunol. 2020. PMID: 33072137 Free PMC article. Review.
-
Cytokines and metabolic factors regulate tumoricidal T-cell function during cancer immunotherapy.Immunotherapy. 2017 Jan;9(1):71-82. doi: 10.2217/imt-2016-0097. Immunotherapy. 2017. PMID: 28000531 Free PMC article. Review.
-
Aberrant Glycosylation of Anchor-Optimized MUC1 Peptides Can Enhance Antigen Binding Affinity and Reverse Tolerance to Cytotoxic T Lymphocytes.Biomolecules. 2016 Jun 29;6(3):31. doi: 10.3390/biom6030031. Biomolecules. 2016. PMID: 27367740 Free PMC article.
-
Enhancing the safety of antibody-based immunomodulatory cancer therapy without compromising therapeutic benefit: Can we have our cake and eat it too?Expert Opin Biol Ther. 2016;16(5):655-74. doi: 10.1517/14712598.2016.1152256. Epub 2016 Feb 25. Expert Opin Biol Ther. 2016. PMID: 26855028 Free PMC article. Review.
-
Central tolerance: what you see is what you don't get!Nat Immunol. 2016 Feb;17(2):115-6. doi: 10.1038/ni.3373. Nat Immunol. 2016. PMID: 26784256 No abstract available.
References
-
- Van Pel, A., P. van der Bruggen, P.G. Coulie, V.G. Brichard, B. Lethe, B. Van den Eynde, C. Uyttenhove, J.-C. Renauld, and T. Boon. 1995. Genes coding for tumor antigens recognized by cytolytic T lymphocytes. Immunol. Rev. 145:229–250. - PubMed
-
- Gilboa, E. 1999. The makings of a tumor rejection antigen. Immunity. 11:263–270. - PubMed
-
- Rosenberg, S.A. 1999. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 10:281–287. - PubMed
-
- Mizoguchi, H., J.J. O'Shea, D.L. Longo, C.M. Loeffler, D.W. McVicar, and A.C. Ochoa. 1992. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 258:1795–1798. - PubMed
-
- Alexander, J.P., S. Kudoh, K.A. Melsop, T.A. Hamilton, M.G. Edinger, R.R. Tubbs, D. Sica, L. Tuason, E. Klein, R.M. Bukowski, et al. 1993. T-cells infiltrating renal cell carcinoma display a poor proliferative response even though they can produce interleukin 2 and express interleukin 2 receptors. Cancer Res. 53:1380–1387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

