Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation
- PMID: 11842121
- PMCID: PMC100354
- DOI: 10.1093/nar/30.4.e15
Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation
Abstract
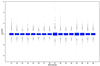
There are many sources of systematic variation in cDNA microarray experiments which affect the measured gene expression levels (e.g. differences in labeling efficiency between the two fluorescent dyes). The term normalization refers to the process of removing such variation. A constant adjustment is often used to force the distribution of the intensity log ratios to have a median of zero for each slide. However, such global normalization approaches are not adequate in situations where dye biases can depend on spot overall intensity and/or spatial location within the array. This article proposes normalization methods that are based on robust local regression and account for intensity and spatial dependence in dye biases for different types of cDNA microarray experiments. The selection of appropriate controls for normalization is discussed and a novel set of controls (microarray sample pool, MSP) is introduced to aid in intensity-dependent normalization. Lastly, to allow for comparisons of expression levels across slides, a robust method based on maximum likelihood estimation is proposed to adjust for scale differences among slides.
Figures


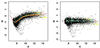


Similar articles
-
A robust neural networks approach for spatial and intensity-dependent normalization of cDNA microarray data.Bioinformatics. 2005 Jun 1;21(11):2674-83. doi: 10.1093/bioinformatics/bti397. Epub 2005 Mar 29. Bioinformatics. 2005. PMID: 15797913
-
A new non-linear normalization method for reducing variability in DNA microarray experiments.Genome Biol. 2002 Aug 30;3(9):research0048. doi: 10.1186/gb-2002-3-9-research0048. Epub 2002 Aug 30. Genome Biol. 2002. PMID: 12225587 Free PMC article.
-
Normalization for two-channel microarray data.Methods Inf Med. 2005;44(3):418-22. Methods Inf Med. 2005. PMID: 16113767
-
Expression profiling of microRNA using real-time quantitative PCR, how to use it and what is available.Methods. 2010 Apr;50(4):244-9. doi: 10.1016/j.ymeth.2010.01.026. Epub 2010 Jan 28. Methods. 2010. PMID: 20109550 Review.
-
Statistical tests for differential expression in cDNA microarray experiments.Genome Biol. 2003;4(4):210. doi: 10.1186/gb-2003-4-4-210. Epub 2003 Mar 17. Genome Biol. 2003. PMID: 12702200 Free PMC article. Review.
Cited by
-
Butyrate induces higher host transcriptional changes to inhibit porcine epidemic diarrhea virus strain CV777 infection in porcine intestine epithelial cells.Virol J. 2024 Jul 11;21(1):157. doi: 10.1186/s12985-024-02428-5. Virol J. 2024. PMID: 38992629 Free PMC article.
-
A systems genomics and genetics approach to identify the genetic regulatory network for lignin content in Brassica napus seeds.Front Plant Sci. 2024 Jun 5;15:1393621. doi: 10.3389/fpls.2024.1393621. eCollection 2024. Front Plant Sci. 2024. PMID: 38903439 Free PMC article.
-
A framework for performance enhancement of classifiers in detection of prostate cancer from microarray gene.Heliyon. 2024 Apr 25;10(9):e29630. doi: 10.1016/j.heliyon.2024.e29630. eCollection 2024 May 15. Heliyon. 2024. PMID: 38720727 Free PMC article.
-
Removing unwanted variation between samples in Hi-C experiments.Brief Bioinform. 2024 Mar 27;25(3):bbae217. doi: 10.1093/bib/bbae217. Brief Bioinform. 2024. PMID: 38711367 Free PMC article.
-
Label-Free Quantitation of Endogenous Peptides.Methods Mol Biol. 2024;2758:125-150. doi: 10.1007/978-1-0716-3646-6_7. Methods Mol Biol. 2024. PMID: 38549012 Free PMC article.
References
-
- Taniguchi M., Miura,K., Iwao,H. and Yamanaka,S. (2001) Quantitative assessment of DNA microarrays—comparison with northern blot analyses. Genomics, 71, 34–39. - PubMed
-
- Hughes T.R., Marton,M.J., Jones,A.R., Roberts,C.J., Stoughton,R., Armour,C.D., Bennett,H.A., Coffey,E., Dai,H., He,Y.D., Kidd,M.J., King,A.M., Meyer,M.R., Slade,D., Lum,P.Y., Stepaniants,S.B., Shoemaker,D.D., Gachotte,D., Chakraburtty,K., Simon,J., Bard,M. and Friend,S.H. (2000) Functional discovery via a compendium of expression profiles. Cell, 102, 109–126. - PubMed
-
- Alizadeh A.A., Eisen,M.B., Davis,R.E., Ma,C., Lossos,I.S., Rosenwald,A., Boldrick,J.C., Sabet,H., Tran,T., Yu,X., Powell,J.I., Yang,L., Marti,G.E., Moore,T., Hudson,J.,Jr, Lu,L., Lewis,D.B., Tibshirani,R., Sherlock,G., Chan,W.C., Greiner,T.C., Weisenburger,D.D., Armitage,J.O., Warnke,R., Staudt,L.M. et al. (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature, 403, 503–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

