Mammalian selenoprotein in which selenocysteine (Sec) incorporation is supported by a new form of Sec insertion sequence element
- PMID: 11839807
- PMCID: PMC134693
- DOI: 10.1128/MCB.22.5.1402-1411.2002
Mammalian selenoprotein in which selenocysteine (Sec) incorporation is supported by a new form of Sec insertion sequence element
Abstract
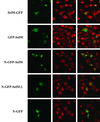
Selenocysteine (Sec), the 21st amino acid in protein, is encoded by UGA. The Sec insertion sequence (SECIS) element, which is the stem-loop structure present in 3' untranslated regions (UTRs) of eukaryotic selenoprotein-encoding genes, is essential for recognition of UGA as a codon for Sec rather than as a stop signal. We now report the identification of a new eukaryotic selenoprotein, designated selenoprotein M (SelM). The 3-kb human SelM-encoding gene has five exons and is located on chromosome 22 but has not been correctly identified by either Celera or the public Human Genome Project. We characterized human and mouse SelM cDNA sequences and expressed the selenoprotein in various mammalian cell lines. The 3" UTR of the human, mouse, and rat SelM-encoding genes lacks a canonical SECIS element. Instead, Sec is incorporated in response to a conserved mRNA structure, in which cytidines are present in place of the adenosines previously considered invariant. Substitution of adenosines for cytidines did not alter Sec incorporation; however, other mutant structures did not support selenoprotein synthesis, demonstrating that this new form of SECIS element is functional. SelM is expressed in a variety of tissues, with increased levels in the brain. It is localized to the perinuclear structures, and its N-terminal signal peptide is necessary for protein translocation.
Figures




Similar articles
-
High-level expression in Escherichia coli of selenocysteine-containing rat thioredoxin reductase utilizing gene fusions with engineered bacterial-type SECIS elements and co-expression with the selA, selB and selC genes.J Mol Biol. 1999 Oct 8;292(5):1003-16. doi: 10.1006/jmbi.1999.3085. J Mol Biol. 1999. PMID: 10512699
-
New mammalian selenocysteine-containing proteins identified with an algorithm that searches for selenocysteine insertion sequence elements.J Biol Chem. 1999 Nov 26;274(48):33888-97. doi: 10.1074/jbc.274.48.33888. J Biol Chem. 1999. PMID: 10567350
-
Characterization of the UGA-recoding and SECIS-binding activities of SECIS-binding protein 2.RNA Biol. 2014;11(11):1402-13. doi: 10.1080/15476286.2014.996472. RNA Biol. 2014. PMID: 25692238 Free PMC article.
-
Mechanism and regulation of selenoprotein synthesis.Annu Rev Nutr. 2003;23:17-40. doi: 10.1146/annurev.nutr.23.011702.073318. Epub 2003 Jan 8. Annu Rev Nutr. 2003. PMID: 12524431 Review.
-
Post-transcriptional control of selenoprotein biosynthesis.Curr Protein Pept Sci. 2012 Jun;13(4):337-46. doi: 10.2174/138920312801619448. Curr Protein Pept Sci. 2012. PMID: 22708491 Review.
Cited by
-
Selenoprotein Gene Nomenclature.J Biol Chem. 2016 Nov 11;291(46):24036-24040. doi: 10.1074/jbc.M116.756155. Epub 2016 Sep 19. J Biol Chem. 2016. PMID: 27645994 Free PMC article.
-
Identification and characterization of Fep15, a new selenocysteine-containing member of the Sep15 protein family.Biochem J. 2006 Mar 15;394(Pt 3):575-9. doi: 10.1042/BJ20051569. Biochem J. 2006. PMID: 16236027 Free PMC article.
-
Isolation and characterization of genes functionally involved in ovarian development of the giant tiger shrimp Penaeus monodon by suppression subtractive hybridization (SSH).Genet Mol Biol. 2010 Oct;33(4):676-85. doi: 10.1590/s1415-47572010000400014. Epub 2010 Dec 1. Genet Mol Biol. 2010. PMID: 21637577 Free PMC article.
-
In vivo neuronal subtype-specific targets of Atoh1 (Math1) in dorsal spinal cord.J Neurosci. 2011 Jul 27;31(30):10859-71. doi: 10.1523/JNEUROSCI.0445-11.2011. J Neurosci. 2011. PMID: 21795538 Free PMC article.
-
Proteomic analysis of kidneys from selenoprotein M transgenic rats in response to increased bioability of selenium.Clin Proteomics. 2013 Aug 12;10(1):10. doi: 10.1186/1559-0275-10-10. Clin Proteomics. 2013. PMID: 23937859 Free PMC article.
References
-
- Atkins, J. F., and R. F. Gesteland. 2000. The twenty-first amino acid. Nature 407:465.. - PubMed
-
- Berry, M. J., G. W. Martin, 3rd, and S. C. Low. 1997. RNA and protein requirements for eukaryotic selenoprotein synthesis. Biomed. Environ. Sci. 10:182-189. - PubMed
-
- Buettner, C., J. W. Harney, and M. J. Berry. 1999. The Caenorhabditis elegans homologue of thioredoxin reductase contains a selenocysteine insertion sequence (SECIS) element that differs from mammalian SECIS elements but directs selenocysteine incorporation. J. Biol. Chem. 274:21598-21602. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
