Centrosome amplification drives chromosomal instability in breast tumor development
- PMID: 11830638
- PMCID: PMC122305
- DOI: 10.1073/pnas.032479999
Centrosome amplification drives chromosomal instability in breast tumor development
Abstract
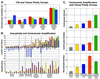
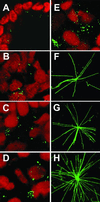
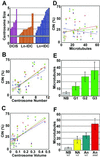
Earlier studies of invasive breast tumors have shown that 60-80% are aneuploid and approximately 80% exhibit amplified centrosomes. In this study, we investigated the relationship of centrosome amplification with aneuploidy, chromosomal instability, p53 mutation, and loss of differentiation in human breast tumors. Twenty invasive breast tumors and seven normal breast tissues were analyzed by fluorescence in situ hybridization with centromeric probes to chromosomes 3, 7, and 17. We analyzed these tumors for both aneuploidy and unstable karyotypes as determined by chromosomal instability. The results were then tested for correlation with three measures of centrosome amplification: centrosome size, centrosome number, and centrosome microtubule nucleation capacity. Centrosome size and centrosome number both showed a positive, significant, linear correlation with aneuploidy and chromosomal instability. Microtubule nucleation capacity showed no such correlation, but did correlate significantly with loss of tissue differentiation. Centrosome amplification was detected in in situ ductal carcinomas, suggesting that centrosome amplification is an early event in these lesions. Centrosome amplification and chromosomal instability occurred independently of p53 mutation, whereas p53 mutation was associated with a significant increase in centrosome microtubule nucleation capacity. Together, these results demonstrate that independent aspects of centrosome amplification correlate with chromosomal instability and loss of tissue differentiation and may be involved in tumor development and progression. These results further suggest that aspects of centrosome amplification may have clinical diagnostic and/or prognostic value and that the centrosome may be a potential target for cancer therapy.
Figures
Similar articles
-
Mammary tumors in mice conditionally mutant for Brca1 exhibit gross genomic instability and centrosome amplification yet display a recurring distribution of genomic imbalances that is similar to human breast cancer.Oncogene. 2002 Aug 1;21(33):5097-107. doi: 10.1038/sj.onc.1205636. Oncogene. 2002. PMID: 12140760
-
Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations.Genes Chromosomes Cancer. 2000 Feb;27(2):183-90. Genes Chromosomes Cancer. 2000. PMID: 10612807 Free PMC article.
-
Simultaneous Aurora-A/STK15 overexpression and centrosome amplification induce chromosomal instability in tumour cells with a MIN phenotype.BMC Cancer. 2007 Nov 13;7:212. doi: 10.1186/1471-2407-7-212. BMC Cancer. 2007. PMID: 17999753 Free PMC article.
-
Centrosome amplification and the origin of chromosomal instability in breast cancer.J Mammary Gland Biol Neoplasia. 2004 Jul;9(3):275-83. doi: 10.1023/B:JOMG.0000048774.27697.30. J Mammary Gland Biol Neoplasia. 2004. PMID: 15557800 Review.
-
Deregulation of the centrosome cycle and the origin of chromosomal instability in cancer.Adv Exp Med Biol. 2005;570:393-421. doi: 10.1007/1-4020-3764-3_14. Adv Exp Med Biol. 2005. PMID: 18727509 Review.
Cited by
-
Aurora B expression and histone variant H1.4S27 phosphorylation are no longer coordinated during metaphase in aneuploid colorectal carcinomas.Virchows Arch. 2015 May;466(5):503-15. doi: 10.1007/s00428-015-1727-6. Epub 2015 Feb 14. Virchows Arch. 2015. PMID: 25680570
-
Centrosomal abnormalities, multipolar mitoses, and chromosomal instability in head and neck tumours with dysfunctional telomeres.Br J Cancer. 2002 Jul 15;87(2):202-7. doi: 10.1038/sj.bjc.6600438. Br J Cancer. 2002. PMID: 12107843 Free PMC article.
-
Genetic, functional, and histopathological evaluation of two C-terminal BRCA1 missense variants.J Med Genet. 2006 Jan;43(1):74-83. doi: 10.1136/jmg.2005.033258. Epub 2005 May 27. J Med Genet. 2006. PMID: 15923272 Free PMC article.
-
Hypoxia Drives Centrosome Amplification in Cancer Cells via HIF1α-dependent Induction of Polo-Like Kinase 4.Mol Cancer Res. 2022 Apr 1;20(4):596-606. doi: 10.1158/1541-7786.MCR-20-0798. Mol Cancer Res. 2022. PMID: 34933912 Free PMC article.
-
Liver kinase B1 regulates the centrosome via PLK1.Cell Death Dis. 2014 Apr 10;5(4):e1157. doi: 10.1038/cddis.2014.135. Cell Death Dis. 2014. PMID: 24722282 Free PMC article.
References
-
- Brinkley B R. Trends Cell Biol. 2001;11:18–21. - PubMed
-
- Lingle W L, Salisbury J L. Curr Top Dev Biol. 2000;49:313–329. - PubMed
-
- Salisbury J L. J Mammary Gland Biol Neoplasia. 2001;6:203–212. - PubMed
-
- Boveri T. Zur Frage der Entstehung Maligner Tumoren. Jena: Fischer; 1914. ; trans. Boveri, M. (1929) The Origin of Malignant Tumors (Williams and Wilkins, Baltimore) (English).
-
- Carroll P E, Okuda M, Horn H F, Biddinger P, Stambrook P J, Gleich L L, Li Y Q, Tarapore P, Fukasawa K. Oncogene. 1999;18:1935–1944. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

