Aging leads to disturbed homeostasis of memory phenotype CD8(+) cells
- PMID: 11828003
- PMCID: PMC2193587
- DOI: 10.1084/jem.20011267
Aging leads to disturbed homeostasis of memory phenotype CD8(+) cells
Abstract
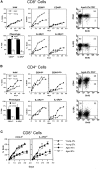
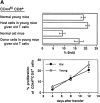
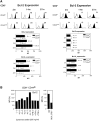
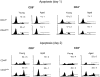
Examining the rate of in vivo T cell turnover (proliferation) in aged mice revealed a marked reduction in turnover at the level of memory-phenotype CD44(hi) CD8(+) cells relative to young mice. Based on adoptive transfer experiments, the reduced turnover of aged CD44(hi) CD8(+) cells reflected an inhibitory influence of the aged host environment. Aged CD44(hi) CD8(+) cells also showed poor in vivo responses to IL-15 and IL-15-inducing agents, but responded well to IL-15 in vitro. Two mechanisms could account for the reduced turnover of aged CD44(hi) CD8(+) cells in vivo. First, aging was associated with a prominent and selective increase in Bcl-2 expression in CD44(hi) CD8(+) cells. Hence, the reduced turnover of aged CD44(hi) CD8(+) cells may in part reflect the antiproliferative effect of enhanced Bcl-2 expression. Second, the impaired in vivo response of aged CD44(hi) CD8(+) cells to IL-15 correlated with increased serum levels of type I interferons (IFN-I) and was largely reversed by injection of anti-IFN-I antibody. Hence the selective reduction in the turnover of aged CD44(hi) CD8(+) cells in vivo may reflect the combined inhibitory effects of enhanced Bcl-2 expression and high IFN-I levels.
Figures







Similar articles
-
IL-15 promotes the survival of naive and memory phenotype CD8+ T cells.J Immunol. 2003 May 15;170(10):5018-26. doi: 10.4049/jimmunol.170.10.5018. J Immunol. 2003. PMID: 12734346
-
CD8+, but not CD4+ effector/memory T cells, express the CD44highCD45RBhigh phenotype with aging, which displays reduced expression levels of P2X7 receptor and ATP-induced cellular responses.FASEB J. 2019 Mar;33(3):3225-3236. doi: 10.1096/fj.201800867R. Epub 2018 Nov 1. FASEB J. 2019. PMID: 30383448
-
Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells.J Exp Med. 2002 Oct 7;196(7):935-46. doi: 10.1084/jem.20020772. J Exp Med. 2002. PMID: 12370255 Free PMC article.
-
Turnover of memory-phenotype CD8+ T cells.Microbes Infect. 2003 Mar;5(3):227-31. doi: 10.1016/s1286-4579(03)00015-7. Microbes Infect. 2003. PMID: 12681412 Review.
-
T-cell turnover in vivo and the role of cytokines.Immunol Lett. 1999 Jan;65(1-2):21-5. doi: 10.1016/s0165-2478(98)00119-9. Immunol Lett. 1999. PMID: 10065622 Review.
Cited by
-
Immunosenescence and organ transplantation.Transplant Rev (Orlando). 2013 Jul;27(3):65-75. doi: 10.1016/j.trre.2013.03.001. Epub 2013 Apr 30. Transplant Rev (Orlando). 2013. PMID: 23639337 Free PMC article. Review.
-
Aging-associated dysregulation of homeostatic immune response termination (and not initiation).Aging Cell. 2017 Jun;16(3):585-593. doi: 10.1111/acel.12589. Epub 2017 Mar 30. Aging Cell. 2017. PMID: 28371013 Free PMC article.
-
Aging and the immune response to organ transplantation.J Clin Invest. 2017 Jun 30;127(7):2523-2529. doi: 10.1172/JCI90601. Epub 2017 May 15. J Clin Invest. 2017. PMID: 28504651 Free PMC article. Review.
-
Mechanisms of severe acute respiratory syndrome pathogenesis and innate immunomodulation.Microbiol Mol Biol Rev. 2008 Dec;72(4):672-85, Table of Contents. doi: 10.1128/MMBR.00015-08. Microbiol Mol Biol Rev. 2008. PMID: 19052324 Free PMC article. Review.
-
Interleukin-21 administration to aged mice rejuvenates their peripheral T-cell pool by triggering de novo thymopoiesis.Aging Cell. 2016 Apr;15(2):349-60. doi: 10.1111/acel.12440. Epub 2016 Jan 13. Aging Cell. 2016. PMID: 26762709 Free PMC article.
References
-
- Hodes, R. 1997. Aging and the immune system. Immunol. Rev. 160:5–8. - PubMed
-
- Miller, R.A., G. Garcia, C.J. Kirk, and J.M. Witkowski. 1997. Early activation defects in T lymphocytes from aged mice. Immunol. Rev. 160:79–90. - PubMed
-
- Linton, P.J., L. Haynes, L. Tsui, X. Zhang, and S. Swain. 1997. From naive to effector—alterations with aging. Immunol. Rev. 160:9–18. - PubMed
-
- Chakravarti, B., and G.N. Abraham. 1999. Aging and T-cell-mediated immunity. Mech. Ageing Dev. 108:183–206. - PubMed
-
- Rosenstein, M.M., and H.R. Strausser. 1980. Macrophage-induced T cell mitogen suppression with age. J. Reticuloendothel. Soc. 27:159–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

