Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism
- PMID: 11826115
- PMCID: PMC6758473
- DOI: 10.1523/JNEUROSCI.22-03-00854.2002
Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism
Abstract
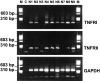
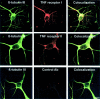
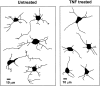
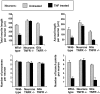
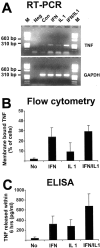
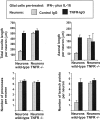
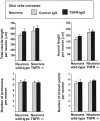
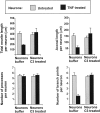
In response to injury and inflammation of the CNS, brain cells including microglia and astrocytes secrete tumor necrosis factor-alpha (TNF). This pro-inflammatory cytokine has been implicated in both neuronal cell death and survival. We now provide evidence that TNF affects the formation of neurites. Neurons cultured on astrocytic glial cells exhibited reduced outgrowth and branching of neurites after addition of recombinant TNF or prestimulation of glial cells to secrete TNF. This effect was absent in neurons of TNF receptor-deficient mice cultured on prestimulated glia of wild-type mice and was reverted by blocking TNF with soluble TNF receptor IgG fusion protein. TNF activated in neurons the small GTPase RhoA. By inactivating Rho with C3 transferase, the inhibitory effect of TNF on neurite outgrowth and branching was abolished. These results suggest that glia-derived TNF, as part of an injury or inflammatory process, can inhibit neurite elongation and branching during development and regeneration.
Figures

Similar articles
-
Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways.J Neurosci. 2002 Apr 15;22(8):3025-32. doi: 10.1523/JNEUROSCI.22-08-03025.2002. J Neurosci. 2002. PMID: 11943805 Free PMC article.
-
Development of a cell transducible RhoA inhibitor TAT-C3 transferase and its encapsulation in biocompatible microspheres to promote survival and enhance regeneration of severed neurons.Pharm Res. 2007 Dec;24(12):2297-308. doi: 10.1007/s11095-007-9454-6. Epub 2007 Sep 25. Pharm Res. 2007. PMID: 17899323
-
Modulation of Rho GTPase activity alleviates chondroitin sulfate proteoglycan-dependent inhibition of neurite extension.J Neurosci Res. 2004 Jul 15;77(2):299-307. doi: 10.1002/jnr.20161. J Neurosci Res. 2004. PMID: 15211597
-
Lithium Distinctly Modulates the Secretion of Pro- and Anti- Inflammatory Interleukins in Co-Cultures of Neurons and Glial Cells at Therapeutic and Sub-Therapeutic Concentrations.Curr Alzheimer Res. 2016;13(8):848-52. doi: 10.2174/1567205013666160219112612. Curr Alzheimer Res. 2016. PMID: 26892291 Review.
-
A study on tumor necrosis factor, tumor necrosis factor receptors, and nitric oxide in human fetal glial cultures.Adv Pharmacol. 1995;34:415-38. doi: 10.1016/s1054-3589(08)61101-1. Adv Pharmacol. 1995. PMID: 8562449 Review. No abstract available.
Cited by
-
Targeted intervention in nerve-cancer crosstalk enhances pancreatic cancer chemotherapy.Nat Nanotechnol. 2024 Nov 4. doi: 10.1038/s41565-024-01803-1. Online ahead of print. Nat Nanotechnol. 2024. PMID: 39496914
-
Regulation of neurite growth by tumour necrosis superfamily member RANKL.Open Biol. 2013 Jan 8;3(1):120150. doi: 10.1098/rsob.120150. Open Biol. 2013. PMID: 23303310 Free PMC article.
-
Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis.J Neurosci. 2004 Mar 17;24(11):2837-45. doi: 10.1523/JNEUROSCI.4789-03.2004. J Neurosci. 2004. PMID: 15028778 Free PMC article.
-
Dual regulation of neuronal morphogenesis by a delta-catenin-cortactin complex and Rho.J Cell Biol. 2003 Jul 7;162(1):99-111. doi: 10.1083/jcb.200211025. Epub 2003 Jun 30. J Cell Biol. 2003. PMID: 12835311 Free PMC article.
-
Managing inflammation after spinal cord injury through manipulation of macrophage function.Neural Plast. 2013;2013:945034. doi: 10.1155/2013/945034. Epub 2013 Oct 31. Neural Plast. 2013. PMID: 24288627 Free PMC article. Review.
References
-
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. - PubMed
-
- Alon R, Cahalon L, Hershkoviz R, Elbaz D, Reizis B, Wallach D, Akiyama SK, Yamada KM, Lider O. TNF-alpha binds to the N-terminal domain of fibronectin and augments the b1-integrin mediated adhesion of CD4+ T lymphocytes to the glycoprotein. J Immunol. 1994;152:1304–1313. - PubMed
-
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM- kinase. Nature. 1998;393:805–809. - PubMed
-
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. - PubMed
-
- Blobel CP. Metalloproteinase disintegrins: links to cell adhesion and cleavage of TNF-alpha and Notch. Cell. 1997;90:589–592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
