The importin-beta binding domain of snurportin1 is responsible for the Ran- and energy-independent nuclear import of spliceosomal U snRNPs in vitro
- PMID: 11815630
- PMCID: PMC2173342
- DOI: 10.1083/jcb.200108114
The importin-beta binding domain of snurportin1 is responsible for the Ran- and energy-independent nuclear import of spliceosomal U snRNPs in vitro
Abstract
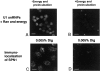
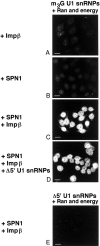
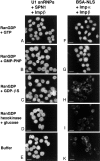
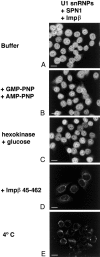
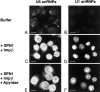
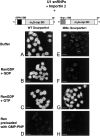
The nuclear localization signal (NLS) of spliceosomal U snRNPs is composed of the U snRNA's 2,2,7-trimethyl-guanosine (m3G)-cap and the Sm core domain. The m3G-cap is specifically bound by snurportin1, which contains an NH2-terminal importin-beta binding (IBB) domain and a COOH-terminal m3G-cap--binding region that bears no structural similarity to known import adaptors like importin-alpha (impalpha). Here, we show that recombinant snurportin1 and importin-beta (impbeta) are not only necessary, but also sufficient for U1 snRNP transport to the nuclei of digitonin-permeabilized HeLa cells. In contrast to impalpha-dependent import, single rounds of U1 snRNP import, mediated by the nuclear import receptor complex snurportin1-impbeta, did not require Ran and energy. The same Ran- and energy-independent import was even observed for U5 snRNP, which has a molecular weight of more than one million. Interestingly, in the presence of impbeta and a snurportin1 mutant containing an impalpha IBB domain (IBBimpalpha), nuclear U1 snRNP import was Ran dependent. Furthermore, beta-galactosidase (betaGal) containing a snurportin1 IBB domain, but not IBBimpalpha-betaGal, was imported into the nucleus in a Ran-independent manner. Our results suggest that the nature of the IBB domain modulates the strength and/or site of interaction of impbeta with nucleoporins of the nuclear pore complex, and thus whether or not Ran is required to dissociate these interactions.
Figures


Comment in
-
Hitchhiking fads en route to peroxisomes.J Cell Biol. 2002 Feb 4;156(3):415-7. doi: 10.1083/jcb.200112122. Epub 2002 Feb 4. J Cell Biol. 2002. PMID: 11827979 Free PMC article. Review.
Similar articles
-
Structural basis for RanGTP independent entry of spliceosomal U snRNPs into the nucleus.J Mol Biol. 2007 Dec 7;374(4):1129-38. doi: 10.1016/j.jmb.2007.09.065. Epub 2007 Sep 29. J Mol Biol. 2007. PMID: 18028944
-
Classical NLS proteins from Saccharomyces cerevisiae.J Mol Biol. 2008 Jun 13;379(4):678-94. doi: 10.1016/j.jmb.2008.04.038. Epub 2008 Apr 22. J Mol Biol. 2008. PMID: 18485366
-
Snurportin1, an m3G-cap-specific nuclear import receptor with a novel domain structure.EMBO J. 1998 Jul 15;17(14):4114-26. doi: 10.1093/emboj/17.14.4114. EMBO J. 1998. PMID: 9670026 Free PMC article.
-
The importin β binding domain as a master regulator of nucleocytoplasmic transport.Biochim Biophys Acta. 2011 Sep;1813(9):1578-92. doi: 10.1016/j.bbamcr.2010.10.012. Epub 2010 Oct 26. Biochim Biophys Acta. 2011. PMID: 21029753 Free PMC article. Review.
-
Structural biology of nucleocytoplasmic transport.Annu Rev Biochem. 2007;76:647-71. doi: 10.1146/annurev.biochem.76.052705.161529. Annu Rev Biochem. 2007. PMID: 17506639 Review.
Cited by
-
Structural basis for m3G-cap-mediated nuclear import of spliceosomal UsnRNPs by snurportin1.EMBO J. 2005 Jul 6;24(13):2235-43. doi: 10.1038/sj.emboj.7600701. Epub 2005 May 26. EMBO J. 2005. PMID: 15920472 Free PMC article.
-
Cross-talk between snurportin1 subdomains.Mol Biol Cell. 2005 Oct;16(10):4660-71. doi: 10.1091/mbc.e05-04-0316. Epub 2005 Jul 19. Mol Biol Cell. 2005. PMID: 16030253 Free PMC article.
-
SMN - A chaperone for nuclear RNP social occasions?RNA Biol. 2017 Jun 3;14(6):701-711. doi: 10.1080/15476286.2016.1236168. Epub 2016 Sep 20. RNA Biol. 2017. PMID: 27648855 Free PMC article. Review.
-
The SMN-ribosome interplay: a new opportunity for Spinal Muscular Atrophy therapies.Biochem Soc Trans. 2024 Feb 28;52(1):465-479. doi: 10.1042/BST20231116. Biochem Soc Trans. 2024. PMID: 38391004 Free PMC article. Review.
-
The importin beta binding domain modulates the avidity of importin beta for the nuclear pore complex.J Biol Chem. 2010 Apr 30;285(18):13769-80. doi: 10.1074/jbc.M109.095760. Epub 2010 Mar 1. J Biol Chem. 2010. PMID: 20197273 Free PMC article.
References
-
- Bayliss, R., T. Littlewood, and M. Stewart. 2000. Structural basis for the interaction between. FxFG nucleoporin repeats and importin-β in nuclear trafficking. Cell. 102:99–108. - PubMed
-
- Cingolani, G., C. Petosa, K. Weis, and C.W. Muller. 1999. Structure of importin-β bound to the IBB domain of importin-α. Nature. 399:221–229. - PubMed
-
- Dickmanns, A., F.R. Bischoff, C. Marshallsay, R. Lührmann, H. Ponstingl, and E. Fanning. 1996. The thermolability of nuclear protein import in tsBN2 cells is suppressed by microinjected Ran-GTP or Ran-GDP, but not by RanQ69L or RanT24N. J. Cell Sci. 109:1449–1457. - PubMed
-
- Englmeier, L., J.C. Olivo, and I.W. Mattaj. 1999. Receptor-mediated substrate translocation through the nuclear pore complex without nucleotide triphosphate hydrolysis. Curr. Biol. 9:30–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

