Actin dependence of polarized receptor recycling in Madin-Darby canine kidney cell endosomes
- PMID: 11809838
- PMCID: PMC65087
- DOI: 10.1091/mbc.01-07-0320
Actin dependence of polarized receptor recycling in Madin-Darby canine kidney cell endosomes
Abstract
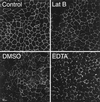
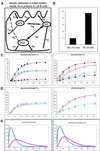
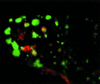
Mammalian epithelial cell plasma membrane domains are separated by junctional complexes supported by actin. The extent to which actin acts elsewhere to maintain cell polarity remains poorly understood. Using latrunculin B (Lat B) to depolymerize actin filaments, several basolateral plasma membrane proteins were found to lose their polarized distribution. This loss of polarity did not reflect lateral diffusion through junctional complexes because a low-density lipoprotein receptor mutant lacking a functional endocytosis signal remained basolateral after Lat B treatment. Furthermore, Lat B treatment did not facilitate membrane diffusion across the tight junction as observed with ethylenediaminetetraacetic acid or dimethyl sulfoxide treatment. Detailed analysis of transferrin recycling confirmed Lat B depolarized recycling of transferrin from endosomes to the basolateral surface. Kinetic analysis suggested sorting was compromised at both basolateral early endosomes and perinuclear recycling endosomes. Despite loss of function, these two endosome populations remained distinct from each other and from early endosomes labeled by apically internalized ligand. Furthermore, apical and basolateral early endosomes were functionally distinct populations that directed traffic to a single common recycling endosomal compartment even after Lat B treatment. Thus, filamentous actin may help to guide receptor traffic from endosomes to the basolateral plasma membrane.
Figures




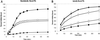

Similar articles
-
The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions.J Cell Biol. 1999 Apr 5;145(1):123-39. doi: 10.1083/jcb.145.1.123. J Cell Biol. 1999. PMID: 10189373 Free PMC article.
-
Sorting of membrane and fluid at the apical pole of polarized Madin-Darby canine kidney cells.Mol Biol Cell. 2000 Jun;11(6):2131-50. doi: 10.1091/mbc.11.6.2131. Mol Biol Cell. 2000. PMID: 10848634 Free PMC article.
-
Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase RhoA.Mol Biol Cell. 1999 Dec;10(12):4369-84. doi: 10.1091/mbc.10.12.4369. Mol Biol Cell. 1999. PMID: 10588664 Free PMC article.
-
The subapical compartment: a traffic center in membrane polarity development.J Cell Sci. 2004 May 1;117(Pt 11):2183-92. doi: 10.1242/jcs.01217. J Cell Sci. 2004. PMID: 15126620 Review.
-
Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity.Trends Cell Biol. 2010 Oct;20(10):618-26. doi: 10.1016/j.tcb.2010.08.004. Trends Cell Biol. 2010. PMID: 20833047 Review.
Cited by
-
MICAL-like1 mediates epidermal growth factor receptor endocytosis.Mol Biol Cell. 2011 Sep;22(18):3431-41. doi: 10.1091/mbc.E11-01-0030. Epub 2011 Jul 27. Mol Biol Cell. 2011. PMID: 21795389 Free PMC article.
-
Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome.Mol Biol Cell. 2008 May;19(5):2059-68. doi: 10.1091/mbc.e07-09-0902. Epub 2008 Feb 20. Mol Biol Cell. 2008. PMID: 18287531 Free PMC article.
-
Vectorial insertion of apical and basolateral membrane proteins in polarized epithelial cells revealed by quantitative 3D live cell imaging.J Cell Biol. 2006 Mar 27;172(7):1035-44. doi: 10.1083/jcb.200512012. J Cell Biol. 2006. PMID: 16567501 Free PMC article.
-
Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity.Mol Biol Cell. 2006 Feb;17(2):977-89. doi: 10.1091/mbc.e05-05-0462. Epub 2005 Dec 7. Mol Biol Cell. 2006. PMID: 16339081 Free PMC article.
-
Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains.Mol Biol Cell. 2007 Jul;18(7):2687-97. doi: 10.1091/mbc.e05-09-0873. Epub 2007 May 9. Mol Biol Cell. 2007. PMID: 17494872 Free PMC article.
References
-
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. - PubMed
-
- Balda MS, Matter K. Tight junctions. J Cell Sci. 1998;111:541–547. - PubMed
-
- Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. - PubMed
-
- Brown PS, Wang E, Areoti B, Chapin SJ, Mostov KE, Dunn KE. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

