Function of the tetraspanin CD151-alpha6beta1 integrin complex during cellular morphogenesis
- PMID: 11809818
- PMCID: PMC65068
- DOI: 10.1091/mbc.01-10-0481
Function of the tetraspanin CD151-alpha6beta1 integrin complex during cellular morphogenesis
Abstract
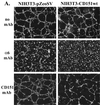
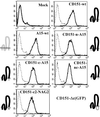
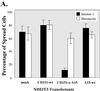
Upon plating on basement membrane Matrigel, NIH3T3 cells formed an anastomosing network of cord-like structures, inhibitable by anti-alpha6beta1 integrin antibodies. For NIH3T3 cells transfected with human CD151 protein, the formation of a cord-like network was also inhibitable by anti-CD151 antibodies. Furthermore, CD151 and alpha6beta1 were physically associated within NIH3T3 cells. On removal of the short 8-amino acid C-terminal CD151 tail (by deletion or exchange), exogenous CD151 exerted a dominant negative effect, as it almost completely suppressed alpha6beta1-dependent cell network formation and NIH3T3 cell spreading on laminin-1 (an alpha6beta1 ligand). Importantly, mutant CD151 retained alpha6beta1 association and did not alter alpha6beta1-mediated cell adhesion to Matrigel. In conclusion, the CD151-alpha6beta1 integrin complex acts as a functional unit that markedly influences cellular morphogenesis, with the CD151 tail being of particular importance in determining the "outside-in" functions of alpha6beta1-integrin that follow ligand engagement. Also, antibodies to alpha6beta1 and CD151 inhibited formation of endothelial cell cord-like networks, thus pointing to possible relevance of CD151-alpha6beta1 complexes during angiogenesis.
Figures







Similar articles
-
Integrin α6β1 Expressed in ESCs Instructs the Differentiation to Endothelial Cells.Stem Cells. 2015 Jun;33(6):1719-29. doi: 10.1002/stem.1974. Stem Cells. 2015. PMID: 25693840 Free PMC article.
-
Tetraspanin CD151 regulates alpha6beta1 integrin adhesion strengthening.Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7616-21. doi: 10.1073/pnas.1337546100. Epub 2003 Jun 12. Proc Natl Acad Sci U S A. 2003. PMID: 12805567 Free PMC article.
-
Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo.J Cell Sci. 2002 Mar 15;115(Pt 6):1161-73. doi: 10.1242/jcs.115.6.1161. J Cell Sci. 2002. PMID: 11884516
-
Tetraspanin protein contributions to cancer.Biochem Soc Trans. 2011 Apr;39(2):547-52. doi: 10.1042/BST0390547. Biochem Soc Trans. 2011. PMID: 21428937 Review.
-
Integrin signaling in leukocytes: lessons from the alpha6beta1 integrin.J Leukoc Biol. 1997 Apr;61(4):397-407. doi: 10.1002/jlb.61.4.397. J Leukoc Biol. 1997. PMID: 9103225 Review.
Cited by
-
CD151 restricts the α6 integrin diffusion mode.J Cell Sci. 2012 Mar 15;125(Pt 6):1478-87. doi: 10.1242/jcs.093963. Epub 2012 Feb 10. J Cell Sci. 2012. PMID: 22328509 Free PMC article.
-
Specific tetraspanin functions.J Cell Biol. 2001 Dec 24;155(7):1103-7. doi: 10.1083/jcb.200108061. Epub 2001 Dec 24. J Cell Biol. 2001. PMID: 11756464 Free PMC article. Review.
-
alpha3beta1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell-cell adhesion.J Cell Biol. 2003 Dec 22;163(6):1351-62. doi: 10.1083/jcb.200306067. J Cell Biol. 2003. PMID: 14691142 Free PMC article.
-
Targeting CD151 by lentivirus-mediated RNA interference inhibits luminal and basal-like breast cancer cell growth and invasion.Mol Cell Biochem. 2015 Sep;407(1-2):111-21. doi: 10.1007/s11010-015-2459-2. Epub 2015 May 24. Mol Cell Biochem. 2015. PMID: 26003442
-
Migrasome and Tetraspanins in Vascular Homeostasis: Concept, Present, and Future.Front Cell Dev Biol. 2020 Jun 16;8:438. doi: 10.3389/fcell.2020.00438. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32612990 Free PMC article. Review.
References
-
- Bauer J, Margolis M, Schreiner C, Edgell CJ, Azizkhan J, Lazarowski E, Juliano RL. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: contributions of induced gene expression, G-proteins, and integrins. J Cell Physiol. 1992;153:437–449. - PubMed
-
- Berdichevsky F, Alford D, D'Souza B, Taylor-Papadimitriou J. Branching morphogenesis of human mammary epithelial cells in collagen gels. J Cell Sci. 1994;107:3557–3568. - PubMed
-
- Berditchevski F, Chang S, Bodorova J, Hemler ME. Generation of monoclonal antibodies to integrin-associated proteins: evidence that α3β1 complexes with EMMPRIN/basigin/OX47/M6. J Biol Chem. 1997;272:29174–29180. - PubMed
-
- Bikfalvi A, Cramer EM, Tenza D, Tobelem G. Phenotypic modulations of human umbilical vein endothelial cells and human dermal fibroblasts using two angiogenic assays. Biol Cell. 1991;72:275–278. - PubMed
-
- Davis GE, Camarillo CW. Regulation of endothelial cell morphogenesis by integrins, mechanical forces, and matrix guidance pathways. Exp Cell Res. 1995;216:113–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

