Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns
- PMID: 11807368
- PMCID: PMC1422424
- DOI: 10.1097/00000658-200202000-00016
Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns
Abstract
Objective: Comparison of cultured skin substitutes (CSS) and split-thickness skin autograft (AG) was performed to assess whether donor-site harvesting can be reduced quantitatively and whether functional and cosmetic outcome is similar qualitatively in the treatment of patients with massive cutaneous burns.
Summary background data: Cultured skin substitutes consisting of collagen-glycosaminoglycan substrates populated with autologous fibroblasts and keratinocytes have been shown to close full-thickness skin wounds in preclinical and clinical studies with acceptable functional and cosmetic results.
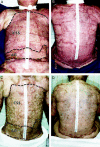
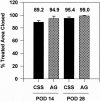
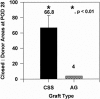
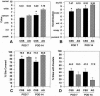
Methods: Qualitative outcome was compared between CSS and AG in 45 patients on an ordinal scale (0, worst; 10, best) with primary analyses at postoperative day 28 and after about 1 year for erythema, pigmentation, pliability, raised scar, epithelial blistering, and surface texture. In the latest 12 of the 45 patients, tracings were performed of donor skin biopsies and wounds treated with CSS at postoperative days 14 and 28 to calculate percentage engraftment, the ratio of closed wound:donor skin areas, and the percentage of total body surface area closed with CSS.
Results: Measures of qualitative outcome of CSS or AG were not different statistically at 1 year after grafting. Engraftment at postoperative day 14 exceeded 75% in the 12 patients evaluated. The ratio of closed wound:donor skin areas for CSS at postoperative day 28 was significantly greater than for conventional 4:1 meshed autografts. The percentage of total body surface area closed with CSS at postoperative day 28 was significantly less than with AG.
Conclusions: The requirement for harvesting of donor skin for CSS was less than for conventional skin autografts. These results suggest that acute-phase recovery of patients with extensive burns is facilitated and that complications are reduced by the use of CSS together with conventional skin grafting.
Figures



Similar articles
-
Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns.J Trauma. 2006 Apr;60(4):821-9. doi: 10.1097/01.ta.0000196802.91829.cc. J Trauma. 2006. PMID: 16612303 Clinical Trial.
-
The 1999 clinical research award. Cultured skin substitutes combined with Integra Artificial Skin to replace native skin autograft and allograft for the closure of excised full-thickness burns.J Burn Care Rehabil. 1999 Nov-Dec;20(6):453-61. doi: 10.1097/00004630-199920060-00006. J Burn Care Rehabil. 1999. PMID: 10613682
-
Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns.Ann Surg. 1995 Dec;222(6):743-52. doi: 10.1097/00000658-199512000-00008. Ann Surg. 1995. PMID: 8526581 Free PMC article. Clinical Trial.
-
Principles and practices for treatment of cutaneous wounds with cultured skin substitutes.Am J Surg. 2002 Apr;183(4):445-56. doi: 10.1016/s0002-9610(02)00813-9. Am J Surg. 2002. PMID: 11975935 Review.
-
Closure of the excised burn wound: autografts, semipermanent skin substitutes, and permanent skin substitutes.Clin Plast Surg. 2009 Oct;36(4):643-51. doi: 10.1016/j.cps.2009.05.010. Clin Plast Surg. 2009. PMID: 19793558 Review.
Cited by
-
Quantitative Evaluation of Human Umbilical Vein and Induced Pluripotent Stem Cell-Derived Endothelial Cells as an Alternative Cell Source to Skin-Specific Endothelial Cells in Engineered Skin Grafts.Adv Wound Care (New Rochelle). 2021 Sep;10(9):490-502. doi: 10.1089/wound.2020.1163. Epub 2020 Sep 17. Adv Wound Care (New Rochelle). 2021. PMID: 32870778 Free PMC article.
-
"Trooping the color": restoring the original donor skin color by addition of melanocytes to bioengineered skin analogs.Pediatr Surg Int. 2013 Mar;29(3):239-47. doi: 10.1007/s00383-012-3217-0. Epub 2012 Nov 30. Pediatr Surg Int. 2013. PMID: 23196807
-
Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice.FASEB J. 2002 Jun;16(8):797-804. doi: 10.1096/fj.01-0868com. FASEB J. 2002. PMID: 12039861 Free PMC article.
-
Vascular endothelial growth factor overexpression increases vascularization by murine but not human endothelial cells in cultured skin substitutes grafted to athymic mice.J Burn Care Rehabil. 2004 Jul-Aug;25(4):337-45. doi: 10.1097/01.bcr.0000132168.02947.a1. J Burn Care Rehabil. 2004. PMID: 15247832 Free PMC article.
-
Long-term expression pattern of melanocyte markers in light- and dark-pigmented dermo-epidermal cultured human skin substitutes.Pediatr Surg Int. 2015 Jan;31(1):69-76. doi: 10.1007/s00383-014-3622-7. Epub 2014 Oct 18. Pediatr Surg Int. 2015. PMID: 25326121
References
-
- Herndon DN, Muller MJ, Blakeney PE. Teamwork for total burn care: achievements, directions and hopes. In Herndon DN, ed. Total burn care. Philadelphia: WB Saunders; 1996: 1–4.
-
- Hansbrough JF, Dominic W, Gadd M, et al. Burns: critical decisions. Prob Crit Care 1987; 1: 558–610.
-
- Burke JF, Quinby WC, Bondoc CC. Early excision and prompt wound closure supplemented with immunosuppression. Surg Clin North Am 1978; 58: 1141–1150. - PubMed
-
- Hansbrough JF, Mozingo DW, Kealey GP, et al. Clinical trials of a biosynthetic temporary skin replacement, Dermagraft-TC compared to cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil 1997; 18: 43–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

