Assembly and function of AP-3 complexes in cells expressing mutant subunits
- PMID: 11807095
- PMCID: PMC2199225
- DOI: 10.1083/jcb.200107140
Assembly and function of AP-3 complexes in cells expressing mutant subunits
Abstract
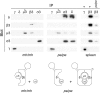
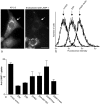
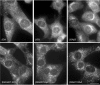
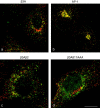
The mouse mutants mocha and pearl are deficient in the AP-3 delta and beta3A subunits, respectively. We have used cells from these mice to investigate both the assembly of AP-3 complexes and AP-3 function. In mocha cells, the beta3 and mu3 subunits coassemble into a heterodimer, whereas the sigma3 subunit remains monomeric. In pearl cells, the delta and sigma3 subunits coassemble into a heterodimer, whereas mu3 gets destroyed. The yeast two hybrid system was used to confirm these interactions, and also to demonstrate that the A (ubiquitous) and B (neuronal-specific) isoforms of beta3 and mu3 can interact with each other. Pearl cell lines were generated that express beta3A, beta3B, a beta3Abeta2 chimera, two beta3A deletion mutants, and a beta3A point mutant lacking a functional clathrin binding site. All six constructs assembled into complexes and were recruited onto membranes. However, only beta3A, beta3B, and the point mutant gave full functional rescue, as assayed by LAMP-1 sorting. The beta3Abeta2 chimera and the beta3A short deletion mutant gave partial functional rescue, whereas the beta3A truncation mutant gave no functional rescue. These results indicate that the hinge and/or ear domains of beta3 are important for function, but the clathrin binding site is not needed.
Figures


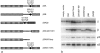
Similar articles
-
Nonsense mutations in ADTB3A cause complete deficiency of the beta3A subunit of adaptor complex-3 and severe Hermansky-Pudlak syndrome type 2.Pediatr Res. 2002 Feb;51(2):150-8. doi: 10.1203/00006450-200202000-00006. Pediatr Res. 2002. PMID: 11809908
-
Intracellular transport of MHC class II and associated invariant chain in antigen presenting cells from AP-3-deficient mocha mice.Cell Immunol. 2001 Jun 15;210(2):143-53. doi: 10.1006/cimm.2001.1817. Cell Immunol. 2001. PMID: 11520080
-
The beta3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness.Hum Mol Genet. 1999 Feb;8(2):323-30. doi: 10.1093/hmg/8.2.323. Hum Mol Genet. 1999. PMID: 9931340
-
Beta3A-adaptin, a subunit of the adaptor-like complex AP-3.J Biol Chem. 1997 Jun 13;272(24):15078-84. doi: 10.1074/jbc.272.24.15078. J Biol Chem. 1997. PMID: 9182526
-
Genetic analyses of adaptin function from yeast to mammals.Gene. 2002 Mar 20;286(2):175-86. doi: 10.1016/s0378-1119(02)00422-5. Gene. 2002. PMID: 11943473 Review.
Cited by
-
SCYL2 Protects CA3 Pyramidal Neurons from Excitotoxicity during Functional Maturation of the Mouse Hippocampus.J Neurosci. 2015 Jul 22;35(29):10510-22. doi: 10.1523/JNEUROSCI.2056-14.2015. J Neurosci. 2015. PMID: 26203146 Free PMC article.
-
Identification of a homozygous deletion in the AP3B1 gene causing Hermansky-Pudlak syndrome, type 2.Blood. 2006 Jul 1;108(1):362-9. doi: 10.1182/blood-2005-11-4377. Epub 2006 Mar 14. Blood. 2006. PMID: 16537806 Free PMC article.
-
Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments.Proc Natl Acad Sci U S A. 2005 May 31;102(22):7910-5. doi: 10.1073/pnas.0502206102. Epub 2005 May 23. Proc Natl Acad Sci U S A. 2005. PMID: 15911768 Free PMC article.
-
Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins.J Cell Biol. 2004 Mar 29;164(7):1065-76. doi: 10.1083/jcb.200311064. J Cell Biol. 2004. PMID: 15051738 Free PMC article.
-
A single β adaptin contributes to AP1 and AP2 complexes and clathrin function in Dictyostelium.Traffic. 2012 Feb;13(2):305-16. doi: 10.1111/j.1600-0854.2011.01310.x. Epub 2011 Dec 4. Traffic. 2012. PMID: 22050483 Free PMC article.
References
-
- Aguilar, R.C., H. Ohno, K.W. Roche, and J.S. Bonifacino. 1997. Functional domain mapping of the clathrin-associated adaptor medium chains μ1 and μ2. J. Biol. Chem. 272:27160–27166. - PubMed
-
- Cowles, C.R., G. Odorizzi, G.S. Payne, and S.D. Emr. 1997. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 91:109–118. - PubMed
-
- Dell'Angelica, E.C., J. Klumperman, W. Stoorvogel, and J.S. Bonifacino. 1998. Association of the AP-3 complex with clathrin. Science. 280:431–434. - PubMed
-
- Dell'Angelica, E.C., V. Shotelersuk, R.C. Aguilar, W.A. Gahl, and J.S. Bonifacino. 1999. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor. Mol. Cell. 3:11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

