A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway
- PMID: 11807092
- PMCID: PMC2199237
- DOI: 10.1083/jcb.200109077
A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway
Abstract
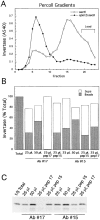
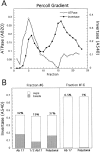
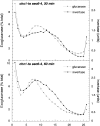
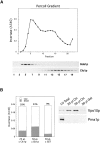
Exocytic vesicles that accumulate in a temperature-sensitive sec6 mutant at a restrictive temperature can be separated into at least two populations with different buoyant densities and unique cargo molecules. Using a sec6 mutant background to isolate vesicles, we have found that vacuolar protein sorting mutants that block an endosome-mediated route to the vacuole, including vps1, pep12, vps4, and a temperature-sensitive clathrin mutant, missort cargo normally transported by dense exocytic vesicles, such as invertase, into light exocytic vesicles, whereas transport of cargo specific to the light exocytic vesicles appears unaffected. Immunoisolation experiments confirm that missorting, rather than a changed property of the normally dense vesicles, is responsible for the altered density gradient fractionation profile. The vps41Delta and apl6Delta mutants, which block transport of only the subset of vacuolar proteins that bypasses endosomes, sort exocytic cargo normally. Furthermore, a vps10Delta sec6 mutant, which lacks the sorting receptor for carboxypeptidase Y (CPY), accumulates both invertase and CPY in dense vesicles. These results suggest that at least one branch of the yeast exocytic pathway transits through endosomes before reaching the cell surface. Consistent with this possibility, we show that immunoisolated clathrin-coated vesicles contain invertase.
Figures






Similar articles
-
Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component.EMBO J. 1997 May 15;16(10):2769-82. doi: 10.1093/emboj/16.10.2769. EMBO J. 1997. PMID: 9184222 Free PMC article.
-
Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment.J Cell Biol. 1993 Jun;121(6):1245-56. doi: 10.1083/jcb.121.6.1245. J Cell Biol. 1993. PMID: 8509446 Free PMC article.
-
The VPS4 gene is involved in protein transport out of a yeast pre-vacuolar endosome-like compartment.Curr Genet. 1997 Jun;31(6):469-80. doi: 10.1007/s002940050232. Curr Genet. 1997. PMID: 9211789
-
Vacuolar sorting. Tracking down an elusive receptor.Curr Biol. 1994 Nov 1;4(11):1019-22. doi: 10.1016/s0960-9822(00)00231-1. Curr Biol. 1994. PMID: 7874484 Review.
-
Receptor-mediated sorting of soluble vacuolar proteins: myths, facts, and a new model.J Exp Bot. 2016 Aug;67(15):4435-49. doi: 10.1093/jxb/erw222. Epub 2016 Jun 4. J Exp Bot. 2016. PMID: 27262127 Review.
Cited by
-
Lipid Exchangers: Cellular Functions and Mechanistic Links With Phosphoinositide Metabolism.Front Cell Dev Biol. 2020 Jul 21;8:663. doi: 10.3389/fcell.2020.00663. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32793602 Free PMC article. Review.
-
Role of the ESCRT Pathway in Laccase Trafficking and Virulence of Cryptococcus neoformans.Infect Immun. 2020 Jun 22;88(7):e00954-19. doi: 10.1128/IAI.00954-19. Print 2020 Jun 22. Infect Immun. 2020. PMID: 32284371 Free PMC article.
-
The Role of Secretory Pathways in Candida albicans Pathogenesis.J Fungi (Basel). 2020 Feb 24;6(1):26. doi: 10.3390/jof6010026. J Fungi (Basel). 2020. PMID: 32102426 Free PMC article. Review.
-
Disruption of vacuolar protein sorting components of the HOPS complex leads to enhanced secretion of recombinant proteins in Pichia pastoris.Microb Cell Fact. 2019 Jul 3;18(1):119. doi: 10.1186/s12934-019-1155-4. Microb Cell Fact. 2019. PMID: 31269943 Free PMC article.
-
Budding Yeast Has a Minimal Endomembrane System.Dev Cell. 2018 Jan 8;44(1):56-72.e4. doi: 10.1016/j.devcel.2017.12.014. Epub 2018 Jan 8. Dev Cell. 2018. PMID: 29316441 Free PMC article.
References
-
- Ausubel, F.M., R. Brent, R. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1987. Current Protocols in Molecular Biology. John Wiley & Sons Inc., New York.
-
- Bleekemolen, J.E., M. Stein, K. von Figura, J.W. Slot, and H.J. Geuze. 1988. The two mannose 6-phosphate receptors have almost identical subcellular distributions in U937 monocytes. Eur. J. Cell Biol. 47:366–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

