Protein folding mediated by solvation: water expulsion and formation of the hydrophobic core occur after the structural collapse
- PMID: 11805324
- PMCID: PMC117366
- DOI: 10.1073/pnas.022387699
Protein folding mediated by solvation: water expulsion and formation of the hydrophobic core occur after the structural collapse
Abstract
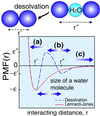
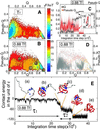
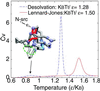
The interplay between structure-search of the native structure and desolvation in protein folding has been explored using a minimalist model. These results support a folding mechanism where most of the structural formation of the protein is achieved before water is expelled from the hydrophobic core. This view integrates water expulsion effects into the funnel energy landscape theory of protein folding. Comparisons to experimental results are shown for the SH3 protein. After the folding transition, a near-native intermediate with partially solvated hydrophobic core is found. This transition is followed by a final step that cooperatively squeezes out water molecules from the partially hydrated protein core.
Figures
Similar articles
-
Probing the folding free energy landscape of the Src-SH3 protein domain.Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):16064-8. doi: 10.1073/pnas.242293099. Epub 2002 Nov 22. Proc Natl Acad Sci U S A. 2002. PMID: 12446834 Free PMC article.
-
Posttransition state desolvation of the hydrophobic core of the src-SH3 protein domain.Biophys J. 2003 Jul;85(1):61-9. doi: 10.1016/S0006-3495(03)74454-3. Biophys J. 2003. PMID: 12829464 Free PMC article.
-
Temperature dependence of the free energy landscape of the src-SH3 protein domain.Proteins. 2004 May 1;55(2):395-406. doi: 10.1002/prot.20053. Proteins. 2004. PMID: 15048830
-
Recent successes of the energy landscape theory of protein folding and function.Q Rev Biophys. 2005 Nov;38(4):405-10. doi: 10.1017/S0033583505004075. Q Rev Biophys. 2005. PMID: 16934172 Review.
-
Theory of protein folding: the energy landscape perspective.Annu Rev Phys Chem. 1997;48:545-600. doi: 10.1146/annurev.physchem.48.1.545. Annu Rev Phys Chem. 1997. PMID: 9348663 Review.
Cited by
-
Strain-dependent fractional molecular diffusion in humid spider silk fibres.J R Soc Interface. 2016 Sep;13(122):20160506. doi: 10.1098/rsif.2016.0506. J R Soc Interface. 2016. PMID: 27628174 Free PMC article.
-
Kinetic rate constant prediction supports the conformational selection mechanism of protein binding.PLoS Comput Biol. 2012 Jan;8(1):e1002351. doi: 10.1371/journal.pcbi.1002351. Epub 2012 Jan 12. PLoS Comput Biol. 2012. PMID: 22253587 Free PMC article.
-
A hypothesis to reconcile the physical and chemical unfolding of proteins.Proc Natl Acad Sci U S A. 2015 May 26;112(21):E2775-84. doi: 10.1073/pnas.1500352112. Epub 2015 May 11. Proc Natl Acad Sci U S A. 2015. PMID: 25964355 Free PMC article.
-
Hydration of the folding transition state ensemble of a protein.Biochemistry. 2006 Mar 21;45(11):3473-80. doi: 10.1021/bi052638z. Biochemistry. 2006. PMID: 16533028 Free PMC article.
-
Visualizing water molecules in transmembrane proteins using radiolytic labeling methods.Biochemistry. 2010 Feb 9;49(5):827-34. doi: 10.1021/bi901889t. Biochemistry. 2010. PMID: 20047303 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

