Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection
- PMID: 11799170
- PMCID: PMC135882
- DOI: 10.1128/jvi.76.4.1753-1761.2002
Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection
Abstract
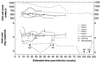
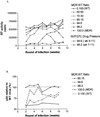
This study examines the persistence and fitness of multidrug-resistant (MDR) viruses acquired during primary human immunodeficiency virus infection (PHI). In four individuals, MDR infections persisted over the entire study period, ranging from 36 weeks to 5 years, in the absence of antiretroviral therapy. In stark contrast, identified source partners in two cases showed expected outgrowth of wild-type (WT) virus within 12 weeks of treatment interruption. In the first PHI case, triple-class MDR resulted in low plasma viremia (1.6 to 3 log copies/ml) over time compared with mean values obtained for an untreated PHI group harboring WT infections (4.1 to 4.3 log copies/ml). Increasing viremia in PHI patient 1 at week 52 was associated with the de novo emergence of a protease inhibitor-resistant variant through a recombination event involving the original MDR virus. MDR infections in two other untreated PHI patients yielded viremia levels typical of the untreated WT group. A fourth patient's MDR infection yielded low viremia (<50 to 500 copies/ml) for 5 years despite his having phenotypic resistance to all antiretroviral drugs in his treatment regimen. In two of these PHI cases, a rebound to higher levels of plasma viremia only occurred when the M184V mutation in reverse transcriptase could no longer be detected and, in a third case, nondetection of M184V was associated with an inability to isolate virus. To further evaluate the fitness of MDR variants acquired in PHI, MDR and corresponding WT viruses were isolated from index and source partners, respectively. Although MDR viral infectivity (50% tissue culture infective dose) was comparable to that observed for WT viruses, MDR infections in each case demonstrated 2-fold and 13- to 23-fold reductions in p24 antigen and reverse transcriptase enzymatic activity, respectively. In dual-infection competition assays, MDR viruses consistently demonstrated a marked replicative disadvantage compared with WT virus. These results indicate that MDR viruses that are generated following PHI can establish persistent infections as dominant quasispecies despite their impaired replicative competence.
Figures
Similar articles
-
Persistence of multidrug-resistant HIV-1 in primary infection leading to superinfection.AIDS. 2004 Aug 20;18(12):1653-60. doi: 10.1097/01.aids.0000131377.28694.04. AIDS. 2004. PMID: 15280776
-
Transmitted human immunodeficiency virus type 1 carrying the D67N or K219Q/E mutation evolves rapidly to zidovudine resistance in vitro and shows a high replicative fitness in the presence of zidovudine.J Virol. 2004 Jul;78(14):7545-52. doi: 10.1128/JVI.78.14.7545-7552.2004. J Virol. 2004. PMID: 15220429 Free PMC article.
-
The HIV-1 Reverse Transcriptase A62V Mutation Influences Replication Fidelity and Viral Fitness in the Context of Multi-Drug-Resistant Mutations.Viruses. 2018 Jul 19;10(7):376. doi: 10.3390/v10070376. Viruses. 2018. PMID: 30029500 Free PMC article.
-
Genotypic resistance tests for the clinical management of patients with primary HIV infection.Scand J Infect Dis Suppl. 2003;106:66-70. Scand J Infect Dis Suppl. 2003. PMID: 15000588 Review.
-
How to win the HIV-1 drug resistance hurdle race: running faster or jumping higher?Biochem J. 2017 Apr 26;474(10):1559-1577. doi: 10.1042/BCJ20160772. Biochem J. 2017. PMID: 28446620 Review.
Cited by
-
Cost-Effectiveness of Antiretroviral Therapy for Multidrug-Resistant HIV: Past, Present, and Future.AIDS Res Treat. 2012;2012:595762. doi: 10.1155/2012/595762. Epub 2012 Nov 8. AIDS Res Treat. 2012. PMID: 23193464 Free PMC article.
-
Criteria for drugs used in pre-exposure prophylaxis trials against HIV infection.PLoS Med. 2006 Nov;3(11):e454. doi: 10.1371/journal.pmed.0030454. PLoS Med. 2006. PMID: 17090213 Free PMC article. Review.
-
Predominance of positive epistasis among drug resistance-associated mutations in HIV-1 protease.PLoS Genet. 2020 Oct 21;16(10):e1009009. doi: 10.1371/journal.pgen.1009009. eCollection 2020 Oct. PLoS Genet. 2020. PMID: 33085662 Free PMC article.
-
The M184V substitution in human immunodeficiency virus type 1 reverse transcriptase delays the development of resistance to amprenavir and efavirenz in subtype B and C clinical isolates.Antimicrob Agents Chemother. 2003 Jul;47(7):2376-9. doi: 10.1128/AAC.47.7.2376-2379.2003. Antimicrob Agents Chemother. 2003. PMID: 12821504 Free PMC article.
-
Amino-acid inserts of HIV-1 capsid (CA) induce CA degradation and abrogate viral infectivity: Insights for the dynamics and mechanisms of HIV-1 CA decomposition.Sci Rep. 2019 Jul 8;9(1):9806. doi: 10.1038/s41598-019-46082-2. Sci Rep. 2019. PMID: 31285456 Free PMC article.
References
-
- Batisse, D., M. Karmochkine, A. Mohamed, C. Piketty, M. D. Kazatchkine, and L. Belec. 1998. Persistence of HIV-1 variants harbouring the zidovudine mutation at pol codon 215 in patients who respond to triple combination therapy. AIDS 12:824-825. - PubMed
-
- Baxter, J. D., D. L. Mayers, D. N. Wentworth, J. D. Neaton, M. L. Hoover, M. A. Winters, S. B. Mannheimer, M. A. Thompson, D. I. Abrams, B. J. Brizz, J. P. Ioannidis, and T. C. Merigan. 2000. A randomized study of antiretroviral management based on plasma genotypic antiretroviral resistance testing in patients failing therapy. CPCRA 046 Study Team for the Terry Beirn Community Programs for Clinical Research on AIDS. AIDS 14:F83-F93. - PubMed
-
- Boden, D., A. Hurley, L. Zhang, Y. Cao, E. Jones, J. Tsay, J. Ip, C. Farthing, K. Limoli, N. Parkin, and M. Markowitz. 1999. HIV-1 drug resistance in newly infected individuals. JAMA 282:1135-1141. - PubMed
-
- Clevenbergh, P., J. Durant, P. Halfon, P. del Giudice, V. Mondain, N. Montagne, J. M. Schapiro, C. A. Boucher, and P. Dellamonica. 2000. Persisting long-term benefit of genotype-guided treatment for HIV-infected patients failing HAART. The Viradapt Study: week 48 follow-up. Antiviral Ther. 5:65-70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

