Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery
- PMID: 11792864
- PMCID: PMC117408
- DOI: 10.1073/pnas.022634999
Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery
Abstract
Dendritic cells (DC) can serve to immunize the newborn immune system to foreign antigen. In a lymphopenic environment, naive T cells undergoing homeostasis-driven proliferation can acquire increased sensitivity to antigen stimulation. Here, we evaluated the capacity of DC to effectively prime the host immune system to elicit antitumor effects in the setting of early lymphoid reconstitution after bone marrow transplantation (BMT). Indeed, bone marrow-derived, cytokine-driven DC pulsed with whole tumor lysates (TP-DC) could, early on, prime a specific and long-lasting antitumor immune response, which mediated the rejection of a lethal challenge of a weakly immunogenic breast tumor. In the therapeutic setting, TP-DC could also inhibit the growth of preexisting breast tumor metastases by repetitive immunizations initiated early after BMT. Spleen T cells obtained from mice immunized with TP-DC early after BMT showed a substantial increase in tumor-specific IFN-gamma production. Our findings demonstrate that it is possible to promote effective antitumor immunity in a defined lymphopenic environment through DC-based immunization.
Figures
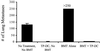
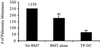
Similar articles
-
Augmentation of antitumor immune responses after adoptive transfer of bone marrow derived from donors immunized with tumor lysate-pulsed dendritic cells.Biol Blood Marrow Transplant. 2004 Aug;10(8):524-33. doi: 10.1016/j.bbmt.2004.04.003. Biol Blood Marrow Transplant. 2004. PMID: 15282530
-
Early vaccination with tumor-lysate-pulsed dendritic cells after allogeneic bone marrow transplantation has antitumor effects.Biol Blood Marrow Transplant. 2006 Oct;12(10):1010-9. doi: 10.1016/j.bbmt.2006.06.009. Biol Blood Marrow Transplant. 2006. PMID: 17084367
-
Increased numbers of monocyte-derived dendritic cells during successful tumor immunotherapy with immune-activating agents.J Immunol. 2013 Aug 15;191(4):1984-92. doi: 10.4049/jimmunol.1301135. Epub 2013 Jul 15. J Immunol. 2013. PMID: 23858033
-
Dendritic cells in antitumor immune responses. II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice, are effective antigen carriers in the therapy of established tumors.Cell Immunol. 1996 May 25;170(1):111-9. doi: 10.1006/cimm.1996.0140. Cell Immunol. 1996. PMID: 8665591
-
[Dendritic cells and the control of the immune response].Pathol Biol (Paris). 2001 Jul;49(6):450-3. doi: 10.1016/s0369-8114(01)00162-6. Pathol Biol (Paris). 2001. PMID: 11484602 Review. French.
Cited by
-
Myeloablative temozolomide enhances CD8⁺ T-cell responses to vaccine and is required for efficacy against brain tumors in mice.PLoS One. 2013;8(3):e59082. doi: 10.1371/journal.pone.0059082. Epub 2013 Mar 18. PLoS One. 2013. PMID: 23527092 Free PMC article.
-
Tumor immunity via homeostatic T cell proliferation: mechanistic aspects and clinical perspectives.Springer Semin Immunopathol. 2005 Jun;27(1):75-85. doi: 10.1007/s00281-004-0196-9. Epub 2005 Jan 22. Springer Semin Immunopathol. 2005. PMID: 15666151 Review.
-
Alkylating chemotherapy may exert a uniquely deleterious effect upon neo-antigen-targeting anticancer vaccination.Oncoimmunology. 2013 Oct 1;2(10):e26294. doi: 10.4161/onci.26294. Epub 2013 Oct 15. Oncoimmunology. 2013. PMID: 24251080 Free PMC article.
-
Depletion of CD4 T cells enhances immunotherapy for neuroblastoma after syngeneic HSCT but compromises development of antitumor immune memory.Blood. 2009 Apr 30;113(18):4449-57. doi: 10.1182/blood-2008-11-190827. Epub 2009 Jan 30. Blood. 2009. PMID: 19182203 Free PMC article.
-
A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer.Clin Exp Med. 2012 Sep;12(3):173-80. doi: 10.1007/s10238-011-0159-0. Epub 2011 Sep 20. Clin Exp Med. 2012. PMID: 21932124 Clinical Trial.
References
-
- Katsanis E, Anderson P M, Filipovich A H, Hasz D E, Rich M L, Loeffler C M, Ochoa A C, Weisdorf D J. Blood. 1991;78:1286–1291. - PubMed
-
- Slavin S, Naparstek E, Nagler A, Ackerstein A, Samual S, Kapelushnik J, Brautbar C, Or R. Blood. 1996;87:2195–2204. - PubMed
-
- Sondak V K, Magner P D, Shu S, Chang A E. Arch Surg. 1991;126:442–446. - PubMed
-
- Finke J H, Zea A H, Stanley J, Longo D L, Ochoa A C. Cancer Res. 1993;53:5613–5616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

