Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis
- PMID: 11784789
- PMCID: PMC6758680
- DOI: 10.1523/JNEUROSCI.22-02-00446.2002
Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis
Abstract
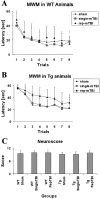
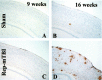
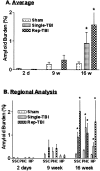
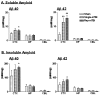
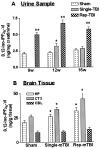
Traumatic brain injury (TBI) increases susceptibility to Alzheimer's disease (AD), but it is not known how TBI contributes to the onset or progression of this common late life dementia. To address this question, we studied neuropathological and behavioral consequences of single versus repetitive mild TBI (mTBI) in transgenic (Tg) mice (Tg2576) that express mutant human Abeta precursor protein, and we demonstrate elevated brain Abeta levels and increased Abeta deposition. Nine-month-old Tg2576 and wild-type mice were subjected to single (n = 15) or repetitive (n = 39) mTBI or sham treatment (n = 37). At 2 d and 9 and 16 weeks after treatment, we assessed brain Abeta deposits and levels in addition to brain and urine isoprostanes generated by lipid peroxidation in these mice. A subset of mice also was studied behaviorally at 16 weeks after injury. Repetitive but not single mTBI increased Abeta deposition as well as levels of Abeta and isoprostanes only in Tg mice, and repetitive mTBI alone induced cognitive impairments but no motor deficits in these mice. This is the first experimental evidence linking TBI to mechanisms of AD by showing that repetitive TBI accelerates brain Abeta accumulation and oxidative stress, which we suggest could work synergistically to promote the onset or drive the progression of AD. Additional insights into the role of TBI in mechanisms of AD pathobiology could lead to strategies for reducing the risk of AD associated with previous episodes of brain trauma and for preventing progressive brain amyloidosis in AD patients.
Figures

Comment in
-
Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury.J Neurochem. 2004 Aug;90(3):758-64. doi: 10.1111/j.1471-4159.2004.02560.x. J Neurochem. 2004. PMID: 15255955
Similar articles
-
Traumatic brain injury precipitates cognitive impairment and extracellular Aβ aggregation in Alzheimer's disease transgenic mice.PLoS One. 2013 Nov 4;8(11):e78851. doi: 10.1371/journal.pone.0078851. eCollection 2013. PLoS One. 2013. PMID: 24223856 Free PMC article.
-
PS2APP transgenic mice, coexpressing hPS2mut and hAPPswe, show age-related cognitive deficits associated with discrete brain amyloid deposition and inflammation.J Neurosci. 2003 Oct 1;23(26):8989-9003. doi: 10.1523/JNEUROSCI.23-26-08989.2003. J Neurosci. 2003. PMID: 14523101 Free PMC article.
-
Apolipoprotein E4 influences amyloid deposition but not cell loss after traumatic brain injury in a mouse model of Alzheimer's disease.J Neurosci. 2002 Dec 1;22(23):10083-7. doi: 10.1523/JNEUROSCI.22-23-10083.2002. J Neurosci. 2002. PMID: 12451108 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Beneficial Effects of Walnuts on Cognition and Brain Health.Nutrients. 2020 Feb 20;12(2):550. doi: 10.3390/nu12020550. Nutrients. 2020. PMID: 32093220 Free PMC article. Review.
Cited by
-
Innate immunity in Alzheimer's disease.Nat Immunol. 2015 Mar;16(3):229-36. doi: 10.1038/ni.3102. Nat Immunol. 2015. PMID: 25689443 Review.
-
The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy.Surg Neurol Int. 2014 Dec 23;5:184. doi: 10.4103/2152-7806.147566. eCollection 2014. Surg Neurol Int. 2014. PMID: 25593768 Free PMC article.
-
BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer's disease.Mol Neurodegener. 2012 Oct 5;7:52. doi: 10.1186/1750-1326-7-52. Mol Neurodegener. 2012. PMID: 23039869 Free PMC article. Review.
-
Protein misassembly and aggregation as potential convergence points for non-genetic causes of chronic mental illness.Mol Psychiatry. 2019 Jul;24(7):936-951. doi: 10.1038/s41380-018-0133-2. Epub 2018 Aug 8. Mol Psychiatry. 2019. PMID: 30089789 Review.
-
Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase.J Neurosci. 2005 Apr 20;25(16):4082-90. doi: 10.1523/JNEUROSCI.4306-04.2005. J Neurosci. 2005. PMID: 15843610 Free PMC article.
References
-
- Bareyre FM, Saatman KE, Raghupathi R, McIntosh TK. Postinjury treatment with magnesium chloride attenuates cortical damage after traumatic brain injury in rats. J Neurotrauma. 2000;17:1029–1039. - PubMed
-
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

