Methanocarba modification of uracil and adenine nucleotides: high potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors
- PMID: 11754592
- PMCID: PMC4957029
- DOI: 10.1021/jm010369e
Methanocarba modification of uracil and adenine nucleotides: high potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors
Abstract
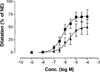
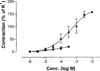
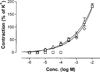
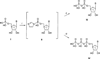
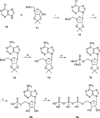
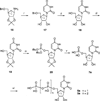
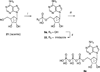
The potency of nucleotide antagonists at P2Y1 receptors was enhanced by replacing the ribose moiety with a constrained carbocyclic ring (Nandanan, et al. J. Med. Chem. 2000, 43, 829-842). We have now synthesized ring-constrained methanocarba analogues (in which a fused cyclopropane moiety constrains the pseudosugar ring) of adenine and uracil nucleotides, the endogenous activators of P2Y receptors. Methanocarba-adenosine 5'-triphosphate (ATP) was fixed in either a Northern (N) or a Southern (S) conformation, as defined in the pseudorotational cycle. (N)-Methanocarba-uridine was prepared from the 1-amino-pseudosugar ring by treatment with beta-ethoxyacryloyl cyanate and cyclization to form the uracil ring. Phosphorylation was carried out at the 5'-hydroxyl group through a multistep process: Reaction with phosphoramidite followed by oxidation provided the 5'-monophosphates, which then were treated with 1,1'-carbonyldiimidazole for condensation with additional phosphate groups. The ability of the analogues to stimulate phospholipase C through activation of turkey P2Y1 or human P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors stably expressed in astrocytoma cells was measured. At recombinant human P2Y1 and P2Y2 receptors, (N)-methanocarba-ATP was 138- and 41-fold, respectively, more potent than racemic (S)-methanocarba-ATP as an agonist. (N)-methanocarba-ATP activated P2Y11 receptors with a potency similar to ATP. (N)-Methanocarba-uridine 5'-triphosphate (UTP) was equipotent to UTP as an agonist at human P2Y2 receptors and also activated P2Y4 receptors with an EC(50) of 85 nM. (N)-Methanocarba-uridine 5'-diphosphate (UDP) was inactive at the hP2Y6 receptor. The vascular effects of (N)-methanocarba-UTP and (N)-methanocarba-UDP were studied in a model of the rat mesenteric artery. The triphosphate was more potent than UTP in inducing a dilatory P2Y4 response (pEC(50) = 6.1 +/- 0.2), while the diphosphate was inactive as either an agonist or antagonist in a P2Y6 receptor-mediated contractile response. Our results suggest that new nucleotide agonists may be designed on the basis of the (N) conformation that favors selectivity for P2Y1, P2Y2, P2Y4, and P2Y11 receptors.
Figures



Similar articles
-
Adenine nucleotide analogues locked in a Northern methanocarba conformation: enhanced stability and potency as P2Y(1) receptor agonists.J Med Chem. 2002 May 9;45(10):2090-100. doi: 10.1021/jm010538v. J Med Chem. 2002. PMID: 11985476 Free PMC article.
-
Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y(1) receptor ligands.J Med Chem. 2000 Mar 9;43(5):829-42. doi: 10.1021/jm990249v. J Med Chem. 2000. PMID: 10715151 Free PMC article.
-
Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors.J Med Chem. 2006 Nov 30;49(24):7076-87. doi: 10.1021/jm060848j. J Med Chem. 2006. PMID: 17125260
-
Molecular pharmacology of P2Y-receptors.Naunyn Schmiedebergs Arch Pharmacol. 2000 Nov;362(4-5):310-23. doi: 10.1007/s002100000310. Naunyn Schmiedebergs Arch Pharmacol. 2000. PMID: 11111826 Review.
-
Pharmacological profiles of cloned mammalian P2Y-receptor subtypes.Pharmacol Ther. 2006 Jun;110(3):415-32. doi: 10.1016/j.pharmthera.2005.08.014. Epub 2005 Oct 28. Pharmacol Ther. 2006. PMID: 16257449 Review.
Cited by
-
Shift in purine/pyrimidine base recognition upon exchanging extracellular domains in P2Y 1/6 chimeric receptors.Biochem Pharmacol. 2004 Nov 15;68(10):2075-86. doi: 10.1016/j.bcp.2004.07.014. Biochem Pharmacol. 2004. PMID: 15476678 Free PMC article.
-
Structure-activity relationship of uridine 5'-diphosphoglucose analogues as agonists of the human P2Y14 receptor.J Med Chem. 2007 May 3;50(9):2030-9. doi: 10.1021/jm061222w. Epub 2007 Apr 4. J Med Chem. 2007. PMID: 17407275 Free PMC article.
-
Anti-photoaging properties of the phosphodiesterase 3 inhibitor cilostazol in ultraviolet B-irradiated hairless mice.Sci Rep. 2016 Aug 3;6:31169. doi: 10.1038/srep31169. Sci Rep. 2016. PMID: 27484958 Free PMC article.
-
Structure activity and molecular modeling analyses of ribose- and base-modified uridine 5'-triphosphate analogues at the human P2Y2 and P2Y4 receptors.Biochem Pharmacol. 2006 Feb 14;71(4):540-9. doi: 10.1016/j.bcp.2005.11.010. Epub 2005 Dec 15. Biochem Pharmacol. 2006. PMID: 16359641 Free PMC article.
-
International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy.Pharmacol Rev. 2006 Sep;58(3):281-341. doi: 10.1124/pr.58.3.3. Pharmacol Rev. 2006. PMID: 16968944 Free PMC article. Review.
References
-
- North RA, Barnard EA. Nucleotide receptors. Curr. Opin. Neurobiol. 1997;7:346–357. - PubMed
-
- King BF, Townsend-Nicholson A. Recombinant P2Y receptors: the UCL experience. J. Auton. Nerv. Syst. 2000;81:164–170. - PubMed
-
- Jacobson KA, Kim Y-C, Camaioni E, van Rhee AM. Structure activity relationships of P2 receptor agonists and antagonists. Chapter 4. In: Turner JT, Weisman G, Fedan J, editors. The P2 Nucleotide Receptors. Clifton, NJ: The Receptors; Humana Press; 1997. pp. 81–107.
-
- Bhagwhat SS, Williams M. P2 Purine and Pyrimidine Receptors: Emerging superfamilies of G protein and ligand-gated ion channel receptors. Eur. J. Med. Chem. 1997;32:183–193.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

