The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells
- PMID: 11748175
- PMCID: PMC127600
- DOI: 10.1128/IAI.70.1.140-146.2002
The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells
Abstract
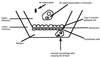
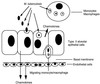
The mechanism(s) by which Mycobacterium tuberculosis crosses the alveolar wall to establish infection in the lung is not well known. In an attempt to better understand the mechanism of translocation and create a model to study the different stages of bacterial crossing through the alveolar wall, we established a two-layer transwell system. M. tuberculosis H37Rv was evaluated regarding the ability to cross and disrupt the membrane. M. tuberculosis invaded A549 type II alveolar cells with an efficiency of 2 to 3% of the initial inoculum, although it was not efficient in invading endothelial cells. However, bacteria that invaded A549 cells were subsequently able to be taken up by endothelial cells with an efficiency of 5 to 6% of the inoculum. When incubated with a bicellular transwell monolayer (epithelial and endothelial cells), M. tuberculosis translocated into the lower chamber with efficiency (3 to 4%). M. tuberculosis was also able to efficiently translocate across the bicellular layer when inside monocytes. Infected monocytes crossed the barrier with greater efficiency when A549 alveolar cells were infected with M. tuberculosis than when A549 cells were not infected. We identified two potential mechanisms by which M. tuberculosis gains access to deeper tissues, by translocating across epithelial cells and by traveling into the blood vessels within monocytes.
Figures
Similar articles
-
Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis.Infect Immun. 1998 Mar;66(3):1121-6. doi: 10.1128/IAI.66.3.1121-1126.1998. Infect Immun. 1998. PMID: 9488404 Free PMC article.
-
Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-kappa B-dependent network.J Immunol. 1999 Oct 1;163(7):3936-47. J Immunol. 1999. PMID: 10490995
-
Induction of nitric oxide release from the human alveolar epithelial cell line A549: an in vitro correlate of innate immune response to Mycobacterium tuberculosis.Immunology. 2004 Jul;112(3):471-80. doi: 10.1046/j.1365-2567.2004.01905.x. Immunology. 2004. PMID: 15196216 Free PMC article.
-
Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells.Front Cell Infect Microbiol. 2019 Aug 21;9:299. doi: 10.3389/fcimb.2019.00299. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31497538 Free PMC article. Review.
-
The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis.Tuberculosis (Edinb). 2010 Nov;90(6):361-6. doi: 10.1016/j.tube.2010.08.005. Epub 2010 Sep 9. Tuberculosis (Edinb). 2010. PMID: 20829117 Review.
Cited by
-
Mycobacterium tuberculosis binding to human surfactant proteins A and D, fibronectin, and small airway epithelial cells under shear conditions.Infect Immun. 2006 Jun;74(6):3587-96. doi: 10.1128/IAI.01644-05. Infect Immun. 2006. PMID: 16714591 Free PMC article.
-
Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish.Cell Host Microbe. 2007 Jul 12;2(1):29-39. doi: 10.1016/j.chom.2007.06.004. Cell Host Microbe. 2007. PMID: 18005715 Free PMC article.
-
Serial measurement of M. tuberculosis in blood from critically-ill patients with HIV-associated tuberculosis.EBioMedicine. 2022 Apr;78:103949. doi: 10.1016/j.ebiom.2022.103949. Epub 2022 Mar 21. EBioMedicine. 2022. PMID: 35325781 Free PMC article.
-
Ultrathin Porated Elastic Hydrogels As a Biomimetic Basement Membrane for Dual Cell Culture.J Vis Exp. 2017 Dec 26;(130):56384. doi: 10.3791/56384. J Vis Exp. 2017. PMID: 29364202 Free PMC article.
-
State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology.ALTEX. 2014;31(4):441-77. doi: 10.14573/altex.1406111. Epub 2014 Jul 14. ALTEX. 2014. PMID: 25027500 Free PMC article. Review.
References
-
- Bermudez, L. E. 1993. Production of transforming growth factor-beta by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-gamma. J. Immunol. 150: 1838–1845. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

