Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast
- PMID: 11739806
- PMCID: PMC60781
- DOI: 10.1091/mbc.12.12.4129
Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast
Abstract
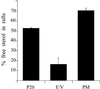
Correct sorting of proteins is essential to generate and maintain the identity and function of the different cellular compartments. In this study we demonstrate the role of lipid rafts in biosynthetic delivery of Pma1p, the major plasma membrane proton ATPase, to the cell surface. Disruption of rafts led to mistargeting of Pma1p to the vacuole. Conversely, Pma1-7, an ATPase mutant that is mistargeted to the vacuole, was shown to exhibit impaired raft association. One of the previously identified suppressors, multicopy AST1, not only restored surface delivery but also raft association of Pma1-7. Ast1p, which is a peripheral membrane protein, was found to directly interact with Pma1p inducing its clustering into a SDS/Triton X100-resistant oligomer. We suggest that clustering facilitates partition of Pma1p into rafts and transport to the cell surface.
Figures
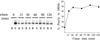
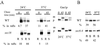

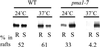





Similar articles
-
Lactoferrin perturbs lipid rafts and requires integrity of Pma1p-lipid rafts association to exert its antifungal activity against Saccharomyces cerevisiae.Int J Biol Macromol. 2021 Feb 28;171:343-357. doi: 10.1016/j.ijbiomac.2020.12.224. Epub 2021 Jan 7. Int J Biol Macromol. 2021. PMID: 33421469
-
Ubiquitin-mediated targeting of a mutant plasma membrane ATPase, Pma1-7, to the endosomal/vacuolar system in yeast.Mol Biol Cell. 2004 May;15(5):2401-9. doi: 10.1091/mbc.e03-10-0727. Epub 2004 Mar 12. Mol Biol Cell. 2004. PMID: 15020711 Free PMC article.
-
Very long-chain fatty acid-containing lipids rather than sphingolipids per se are required for raft association and stable surface transport of newly synthesized plasma membrane ATPase in yeast.J Biol Chem. 2006 Nov 10;281(45):34135-45. doi: 10.1074/jbc.M603791200. Epub 2006 Sep 15. J Biol Chem. 2006. PMID: 16980694
-
Lipid-dependent surface transport of the proton pumping ATPase: a model to study plasma membrane biogenesis in yeast.Biochimie. 2007 Feb;89(2):249-54. doi: 10.1016/j.biochi.2006.07.020. Epub 2006 Aug 15. Biochimie. 2007. PMID: 16938383 Review.
-
The yeast Pma1 proton pump: a model for understanding the biogenesis of plasma membrane proteins.J Biol Chem. 2001 Aug 10;276(32):29613-6. doi: 10.1074/jbc.R100022200. Epub 2001 Jun 12. J Biol Chem. 2001. PMID: 11404364 Review. No abstract available.
Cited by
-
Raft-like membrane domains in pathogenic microorganisms.Curr Top Membr. 2015;75:233-68. doi: 10.1016/bs.ctm.2015.03.005. Epub 2015 Apr 11. Curr Top Membr. 2015. PMID: 26015285 Free PMC article. Review.
-
Arv1 lipid transporter function is conserved between pathogenic and nonpathogenic fungi.Fungal Genet Biol. 2012 Feb;49(2):101-13. doi: 10.1016/j.fgb.2011.11.006. Epub 2011 Nov 27. Fungal Genet Biol. 2012. PMID: 22142782 Free PMC article.
-
Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast.PLoS Biol. 2004 Mar;2(3):E79. doi: 10.1371/journal.pbio.0020079. Epub 2004 Mar 16. PLoS Biol. 2004. PMID: 15024427 Free PMC article.
-
The Oligomeric State of the Plasma Membrane H⁺-ATPase from Kluyveromyces lactis.Molecules. 2019 Mar 8;24(5):958. doi: 10.3390/molecules24050958. Molecules. 2019. PMID: 30857224 Free PMC article.
-
Key role of lipid management in nitrogen and aroma metabolism in an evolved wine yeast strain.Microb Cell Fact. 2016 Feb 9;15:32. doi: 10.1186/s12934-016-0434-6. Microb Cell Fact. 2016. PMID: 26861624 Free PMC article.
References
-
- Ambesi A, Miranda M, Petrov VV, Slayman CW. Biogenesis and function of the yeast plasma-membrane H(+)-ATPase. J Exp Biol. 2000;203(Pt 1):155–160. - PubMed
-
- Benito B, Moreno E, Lagunas R. Half-life of the plasma membrane ATPase and its activating system in resting yeast cells. Biochim Biophys Acta. 1991;1063:265–268. - PubMed
-
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

