Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate
- PMID: 11726523
- PMCID: PMC125328
- DOI: 10.1093/emboj/20.23.6877
Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate
Abstract
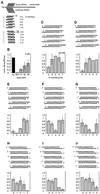
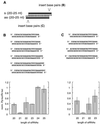
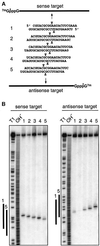
Duplexes of 21-23 nucleotide (nt) RNAs are the sequence-specific mediators of RNA interference (RNAi) and post-transcriptional gene silencing (PTGS). Synthetic, short interfering RNAs (siRNAs) were examined in Drosophila melanogaster embryo lysate for their requirements regarding length, structure, chemical composition and sequence in order to mediate efficient RNAi. Duplexes of 21 nt siRNAs with 2 nt 3' overhangs were the most efficient triggers of sequence-specific mRNA degradation. Substitution of one or both siRNA strands by 2'-deoxy or 2'-O-methyl oligonucleotides abolished RNAi, although multiple 2'-deoxynucleotide substitutions at the 3' end of siRNAs were tolerated. The target recognition process is highly sequence specific, but not all positions of a siRNA contribute equally to target recognition; mismatches in the centre of the siRNA duplex prevent target RNA cleavage. The position of the cleavage site in the target RNA is defined by the 5' end of the guide siRNA rather than its 3' end. These results provide a rational basis for the design of siRNAs in future gene targeting experiments.
Figures

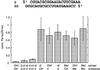

Similar articles
-
RNAi as random degradative PCR: siRNA primers convert mRNA into dsRNAs that are degraded to generate new siRNAs.Cell. 2001 Nov 2;107(3):297-307. doi: 10.1016/s0092-8674(01)00537-2. Cell. 2001. PMID: 11701121
-
RNA interference is mediated by 21- and 22-nucleotide RNAs.Genes Dev. 2001 Jan 15;15(2):188-200. doi: 10.1101/gad.862301. Genes Dev. 2001. PMID: 11157775 Free PMC article.
-
Sequence, chemical, and structural variation of small interfering RNAs and short hairpin RNAs and the effect on mammalian gene silencing.Antisense Nucleic Acid Drug Dev. 2003 Apr;13(2):83-105. doi: 10.1089/108729003321629638. Antisense Nucleic Acid Drug Dev. 2003. PMID: 12804036
-
Gene silencing mediated by small interfering RNAs in mammalian cells.Curr Med Chem. 2003 Feb;10(3):245-56. doi: 10.2174/0929867033368493. Curr Med Chem. 2003. PMID: 12570711 Review.
-
A short primer on RNAi: RNA-directed RNA polymerase acts as a key catalyst.Cell. 2001 Nov 16;107(4):415-8. doi: 10.1016/s0092-8674(01)00581-5. Cell. 2001. PMID: 11719182 Review.
Cited by
-
Rabies Prophylactic and Treatment Options: An In Vitro Study of siRNA- and Aptamer-Based Therapeutics.Viruses. 2021 May 11;13(5):881. doi: 10.3390/v13050881. Viruses. 2021. PMID: 34064911 Free PMC article. Review.
-
The MIDASIN and NOTCHLESS genes are essential for female gametophyte development in Arabidopsis thaliana.Physiol Mol Biol Plants. 2010 Jan;16(1):3-18. doi: 10.1007/s12298-010-0005-y. Epub 2010 Aug 13. Physiol Mol Biol Plants. 2010. PMID: 23572950 Free PMC article.
-
Crystal structure, stability and in vitro RNAi activity of oligoribonucleotides containing the ribo-difluorotoluyl nucleotide: insights into substrate requirements by the human RISC Ago2 enzyme.Nucleic Acids Res. 2007;35(19):6424-38. doi: 10.1093/nar/gkm664. Epub 2007 Sep 18. Nucleic Acids Res. 2007. PMID: 17881374 Free PMC article.
-
High potency silencing by single-stranded boranophosphate siRNA.Nucleic Acids Res. 2006 May 22;34(9):2773-81. doi: 10.1093/nar/gkl339. Print 2006. Nucleic Acids Res. 2006. PMID: 16717282 Free PMC article.
-
Inhibition of interleukin-1beta-induced group IIA secretory phospholipase A2 expression by peroxisome proliferator-activated receptors (PPARs) in rat vascular smooth muscle cells: cooperation between PPARbeta and the proto-oncogene BCL-6.Mol Cell Biol. 2007 Dec;27(23):8374-87. doi: 10.1128/MCB.00623-07. Epub 2007 Oct 1. Mol Cell Biol. 2007. PMID: 17908795 Free PMC article.
References
-
- Ambros V. (2000) Control of developmental timing in Caenorhabditis elegans. Curr. Opin. Genet. Dev., 10, 428–433. - PubMed
-
- Bass B.L. (2000) Double-stranded RNA as a template for gene silencing. Cell, 101, 235–238. - PubMed
-
- Bernstein E., Caudy,A.A., Hammond,S.M. and Hannon,G.J. (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature, 409, 363–366. - PubMed
-
- Carthew R.W. (2001) Gene silencing by double-stranded RNA. Curr. Opin. Cell Biol., 13, 244–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

