Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis
- PMID: 11717450
- PMCID: PMC61145
- DOI: 10.1073/pnas.241377298
Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis
Abstract
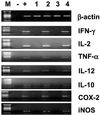
The proteinase-activated receptor 2 (PAR-2) is a member of a family of G protein-coupled receptors for proteases. Proteases cleave PARs within the extracellular N-terminal domains to expose tethered ligands that bind to and activate the cleaved receptors. PAR-2 is highly expressed in colon in epithelial and neuronal elements. In this study we show that PAR-2 activation prevents the development and induces healing of T helper cell type 1-mediated experimental colitis induced by intrarectal administration of 2,4,6-trinitrobenzene sulfonic acid (TNBS) in mice. A role for PAR-2 in the protection against colon inflammation was explored by the use of SLIGRL-NH(2), a synthetic peptide that corresponds to the mouse tethered ligand exposed after PAR-2 cleavage. TNBS-induced colitis was dose-dependently reduced by the administration of SLIGRL-NH(2), whereas the scramble control peptide, LSIGRL-NH(2), was uneffective. This beneficial effect was reflected by increased survival rates, improvement of macroscopic and histologic scores, decrease in mucosal content of T helper cell type 1 cytokines, protein, and mRNA, and a diminished myeloperoxidase activity. SLIGRL-NH(2), but not the scramble peptide, directly inhibited IFN-gamma secretion and CD44 expression on lamina propria T lymphocytes. Protection exerted by PAR-2 in TNBS-treated mice was reverted by injecting mice with a truncated form of calcitonin gene-related peptide and by sensory neurons ablation with the neurotoxin capsaicin. Collectively, these studies show that PAR-2 is an anti-inflammatory receptor in the colon and suggest that PAR-2 ligands might be effective in the treatment of inflammatory bowel diseases.
Figures

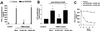
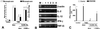
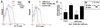
Similar articles
-
A role for proteinase-activated receptor-1 in inflammatory bowel diseases.J Clin Invest. 2004 Nov;114(10):1444-56. doi: 10.1172/JCI21689. J Clin Invest. 2004. Retraction in: J Clin Invest. 2006 Jul;116(7):2056. doi: 10.1172/jci21689r1. PMID: 15545995 Free PMC article. Retracted.
-
Proteinase-activated receptor-4 evoked colorectal analgesia in mice: an endogenously activated feed-back loop in visceral inflammatory pain.Neurogastroenterol Motil. 2012 Jan;24(1):76-85, e13. doi: 10.1111/j.1365-2982.2011.01805.x. Epub 2011 Nov 1. Neurogastroenterol Motil. 2012. PMID: 22044612
-
Protease-activated receptor-2 activation: a major actor in intestinal inflammation.Gut. 2008 Sep;57(9):1222-9. doi: 10.1136/gut.2008.150722. Epub 2008 May 6. Gut. 2008. PMID: 18460552
-
Proteinase-activated receptors: novel mechanisms of signaling by serine proteases.Am J Physiol. 1998 Jun;274(6):C1429-52. doi: 10.1152/ajpcell.1998.274.6.C1429. Am J Physiol. 1998. PMID: 9696685 Review.
-
[PAR (protease-activated receptor) as a novel target for development of gastric mucosal cytoprotective drugs].Nihon Yakurigaku Zasshi. 2002 Nov;120(1):85P-87P. Nihon Yakurigaku Zasshi. 2002. PMID: 12491789 Review. Japanese.
Cited by
-
Protease-Activated Receptors in the Intestine: Focus on Inflammation and Cancer.Front Endocrinol (Lausanne). 2019 Oct 24;10:717. doi: 10.3389/fendo.2019.00717. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31708870 Free PMC article. Review.
-
Suppression of pancreatitis-related allodynia/hyperalgesia by proteinase-activated receptor-2 in mice.Br J Pharmacol. 2006 May;148(1):54-60. doi: 10.1038/sj.bjp.0706708. Br J Pharmacol. 2006. PMID: 16520745 Free PMC article.
-
Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction.Br J Pharmacol. 2008 Mar;153 Suppl 1(Suppl 1):S298-309. doi: 10.1038/sj.bjp.0707508. Epub 2007 Nov 26. Br J Pharmacol. 2008. PMID: 18037927 Free PMC article. Review.
-
NCX-1015, a nitric-oxide derivative of prednisolone, enhances regulatory T cells in the lamina propria and protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice.Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15770-5. doi: 10.1073/pnas.232583599. Epub 2002 Nov 11. Proc Natl Acad Sci U S A. 2002. PMID: 12427966 Free PMC article.
-
Protective effect of proteinase-activated receptor 2 activation on motility impairment and tissue damage induced by intestinal ischemia/reperfusion in rodents.Am J Pathol. 2006 Jul;169(1):177-88. doi: 10.2353/ajpath.2006.051098. Am J Pathol. 2006. PMID: 16816371 Free PMC article.
References
-
- Dery O, Corvera C U, Steinhoff M, Bunnett N W. Am J Physiol. 1998;274:1429–1452. - PubMed
-
- Cirino G, Bucci M, Cicala C, Napoli C. Trends Pharmacol Sci. 2000;21:170–172. - PubMed
-
- Vergnolle N, Wallace J L, Bunnett N W, Hollenberg M D. Trends Pharmacol Sci. 2001;22:146–152. - PubMed
-
- Vu T K, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

