Plasma membrane rafts play a critical role in HIV-1 assembly and release
- PMID: 11717449
- PMCID: PMC61143
- DOI: 10.1073/pnas.241320298
Plasma membrane rafts play a critical role in HIV-1 assembly and release
Abstract
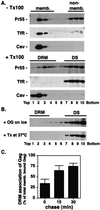
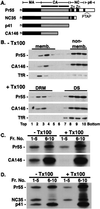
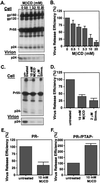
HIV-1 particle production occurs in a series of steps promoted by the viral Gag protein. Although it is well established that assembly and release take place at the plasma membrane, the nature of membrane assembly sites remains poorly understood. We show here that Gag specifically associates with cholesterol-enriched microdomains ("rafts") at the plasma membrane. Kinetic studies demonstrate that raft association follows membrane binding, and the analysis of Gag mutants reveals that, whereas the N terminus of Gag mediates raft binding, this association is greatly enhanced by Gag-Gag interaction domains. We observe that depletion of cellular cholesterol markedly and specifically reduces HIV-1 particle production. Furthermore, treatment of virus-producing cells or virus particles with raft-disrupting agents significantly impairs virus infectivity. These results identify the association of Gag with plasma membrane rafts as an important step in HIV-1 replication. These findings may lead to novel strategies for suppressing HIV-1 replication in vivo.
Figures

Similar articles
-
Human immunodeficiency virus type 1 assembly and lipid rafts: Pr55(gag) associates with membrane domains that are largely resistant to Brij98 but sensitive to Triton X-100.J Virol. 2003 Apr;77(8):4805-17. doi: 10.1128/jvi.77.8.4805-4817.2003. J Virol. 2003. PMID: 12663787 Free PMC article.
-
Depletion of cellular cholesterol inhibits membrane binding and higher-order multimerization of human immunodeficiency virus type 1 Gag.Virology. 2007 Mar 30;360(1):27-35. doi: 10.1016/j.virol.2006.10.011. Epub 2006 Nov 13. Virology. 2007. PMID: 17095032 Free PMC article.
-
Independent segregation of human immunodeficiency virus type 1 Gag protein complexes and lipid rafts.J Virol. 2003 Feb;77(3):1916-26. doi: 10.1128/jvi.77.3.1916-1926.2003. J Virol. 2003. PMID: 12525626 Free PMC article.
-
HIV-1 assembly and maturation.Arch Virol. 2004 Jun;149(6):1067-82. doi: 10.1007/s00705-003-0281-8. Epub 2004 Mar 5. Arch Virol. 2004. PMID: 15168195 Review.
-
[Membrane Binding of Retroviral Gag Proteins].Uirusu. 2014;64(2):155-64. doi: 10.2222/jsv.64.155. Uirusu. 2014. PMID: 26437838 Review. Japanese.
Cited by
-
Functional Dissection of the Dominant Role of CD55 in Protecting Vesicular Stomatitis Virus against Complement-Mediated Neutralization.Viruses. 2021 Feb 26;13(3):373. doi: 10.3390/v13030373. Viruses. 2021. PMID: 33652918 Free PMC article.
-
Regulation of membrane protein structure and function by their lipid nano-environment.Nat Rev Mol Cell Biol. 2023 Feb;24(2):107-122. doi: 10.1038/s41580-022-00524-4. Epub 2022 Sep 2. Nat Rev Mol Cell Biol. 2023. PMID: 36056103 Free PMC article. Review.
-
Loss of Niemann Pick type C proteins 1 and 2 greatly enhances HIV infectivity and is associated with accumulation of HIV Gag and cholesterol in late endosomes/lysosomes.Virol J. 2012 Jan 24;9:31. doi: 10.1186/1743-422X-9-31. Virol J. 2012. PMID: 22273177 Free PMC article.
-
Assembly and architecture of HIV.Adv Exp Med Biol. 2012;726:441-65. doi: 10.1007/978-1-4614-0980-9_20. Adv Exp Med Biol. 2012. PMID: 22297526 Free PMC article. Review.
-
Effect of efavirenz on high-density lipoprotein antioxidant properties in HIV-infected patients.Br J Clin Pharmacol. 2009 Dec;68(6):891-7. doi: 10.1111/j.1365-2125.2009.03535.x. Br J Clin Pharmacol. 2009. PMID: 20002083 Free PMC article.
References
-
- Freed E O. Virology. 1998;251:1–15. - PubMed
-
- Swanstrom R, Wills J W. In: Retroviruses. Coffin J M, Hughes S H, Varmus H E, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 263–334.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

