CD8beta endows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56(lck) complexes
- PMID: 11714755
- PMCID: PMC2193676
- DOI: 10.1084/jem.194.10.1485
CD8beta endows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56(lck) complexes
Abstract
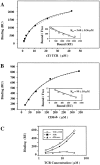
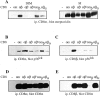
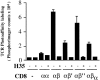
The extraordinary sensitivity of CD8+ T cells to recognize antigen impinges to a large extent on the coreceptor CD8. While several studies have shown that the CD8beta chain endows CD8 with efficient coreceptor function, the molecular basis for this is enigmatic. Here we report that cell-associated CD8alphabeta, but not CD8alphaalpha or soluble CD8alphabeta, substantially increases the avidity of T cell receptor (TCR)-ligand binding. To elucidate how the cytoplasmic and transmembrane portions of CD8beta endow CD8 with efficient coreceptor function, we examined T1.4 T cell hybridomas transfected with various CD8beta constructs. T1.4 hybridomas recognize a photoreactive Plasmodium berghei circumsporozoite (PbCS) peptide derivative (PbCS (4-azidobezoic acid [ABA])) in the context of H-2K(d), and permit assessment of TCR-ligand binding by TCR photoaffinity labeling. We find that the cytoplasmic portion of CD8beta, mainly due to its palmitoylation, mediates partitioning of CD8 in lipid rafts, where it efficiently associates with p56(lck). In addition, the cytoplasmic portion of CD8beta mediates constitutive association of CD8 with TCR/CD3. The resulting TCR-CD8 adducts exhibit high affinity for major histocompatibility complex (MHC)-peptide. Importantly, because CD8alphabeta partitions in rafts, its interaction with TCR/CD3 promotes raft association of TCR/CD3. Engagement of these TCR/CD3-CD8/lck adducts by multimeric MHC-peptide induces activation of p56(lck) in rafts, which in turn phosphorylates CD3 and initiates T cell activation.
Figures




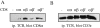


Similar articles
-
Essential role of CD8 palmitoylation in CD8 coreceptor function.J Immunol. 2000 Aug 15;165(4):2068-76. doi: 10.4049/jimmunol.165.4.2068. J Immunol. 2000. PMID: 10925291
-
CTL activation is induced by cross-linking of TCR/MHC-peptide-CD8/p56lck adducts in rafts.Eur J Immunol. 2001 May;31(5):1561-70. doi: 10.1002/1521-4141(200105)31:5<1561::AID-IMMU1561>3.0.CO;2-W. Eur J Immunol. 2001. PMID: 11465114
-
CD3 delta establishes a functional link between the T cell receptor and CD8.J Biol Chem. 2003 Jan 31;278(5):3257-64. doi: 10.1074/jbc.M208119200. Epub 2002 Sep 4. J Biol Chem. 2003. PMID: 12215456
-
Selected signalling proteins recruited to the T-cell receptor-CD3 complex.Immunology. 2018 Jan;153(1):42-50. doi: 10.1111/imm.12809. Epub 2017 Sep 5. Immunology. 2018. PMID: 28771705 Free PMC article. Review.
-
Lipid rafts in T cell receptor signalling.Mol Membr Biol. 2006 Jan-Feb;23(1):49-57. doi: 10.1080/09687860500453673. Mol Membr Biol. 2006. PMID: 16611580 Free PMC article. Review.
Cited by
-
Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle.Proc Natl Acad Sci U S A. 2016 Jun 14;113(24):6677-82. doi: 10.1073/pnas.1602875113. Epub 2016 May 31. Proc Natl Acad Sci U S A. 2016. PMID: 27247384 Free PMC article.
-
Single-cell transcriptomics identifies multiple pathways underlying antitumor function of TCR- and CD8αβ-engineered human CD4+ T cells.Sci Adv. 2020 Jul 3;6(27):eaaz7809. doi: 10.1126/sciadv.aaz7809. eCollection 2020 Jul. Sci Adv. 2020. PMID: 32923584 Free PMC article.
-
Co-receptors and recognition of self at the immunological synapse.Curr Top Microbiol Immunol. 2010;340:171-89. doi: 10.1007/978-3-642-03858-7_9. Curr Top Microbiol Immunol. 2010. PMID: 19960314 Free PMC article. Review.
-
Efficacy against Lung Cancer Is Augmented by Combining Aberrantly N-Glycosylated T Cells with a Chimeric Antigen Receptor Targeting Fragile X Mental Retardation 1 Neighbor.J Immunol. 2024 Mar 1;212(5):917-927. doi: 10.4049/jimmunol.2300618. J Immunol. 2024. PMID: 38214607 Free PMC article.
-
CD8 kinetically promotes ligand binding to the T-cell antigen receptor.Biophys J. 2005 Sep;89(3):2121-33. doi: 10.1529/biophysj.105.061671. Epub 2005 Jun 24. Biophys J. 2005. PMID: 15980174 Free PMC article.
References
-
- Janeway, C.A., Jr. 1992. The T cell receptor as a multicomponent signaling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu. Rev. Immunol. 10:645–674. - PubMed
-
- Zamoyska, R. 1994. The CD8 coreceptor revisited: one chain good, two chains better. Immunity. 1:243–246. - PubMed
-
- Wheeler, C.J., J.-Y. Chen, T.A. Potter, and J.R. Potter. 1998. Mechanism of CD8β-mediated T cell response enhancement: interaction with MHC class I-β2-microglobulin and functional coupling to TCR/CD3. J. Immunol. 160:4199–4207. - PubMed
-
- Bosselut, R., S. Kubo, T. Guinter, J.L. Kopacz, J.D. Altman, L. Feigenbaum, and A. Singer. 2000. Role of CD8beta domains in CD8 coreceptor function: importance for MHC I binding, signaling, and positive selection of CD8+ T cells in the thymus. Immunity. 12:409–418. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

