Damage-induced Bax N-terminal change, translocation to mitochondria and formation of Bax dimers/complexes occur regardless of cell fate
- PMID: 11707402
- PMCID: PMC125731
- DOI: 10.1093/emboj/20.22.6306
Damage-induced Bax N-terminal change, translocation to mitochondria and formation of Bax dimers/complexes occur regardless of cell fate
Abstract
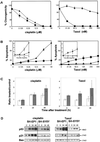
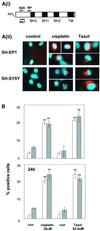
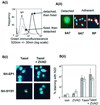
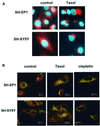
Sequential steps in the activation of the pro-apoptotic protein Bax are described for cells with different sensitivity to cytotoxins. SH-EP1 and SH-SY5Y human neuroblastoma cells, derived from a single precursor cell line, differed in their sensitivity to taxol but showed the same sensitivity to cisplatin. Both drugs, in both cell lines, induced exposure of a constitutively occluded N-terminal epitope of Bax. This was reversible and occurred before the translocation of cytosolic Bax to mitochondria. The N-terminal change in Bax, its subsequent movement to mitochondria and its dimerization/complex formation were insufficient for commitment to death, occurring in the same proportion of cells that either maintained (SH-SY5Y) or lost (SH-EP1) clonogenic survival after taxol treatment. Suppression of taxol-induced apoptosis occurred upstream of cytochrome c release from mitochondria in SH-SY5Y cells. The data suggest that a further drug damage-induced event occurs after Bax dimerization/complex formation but prior to cytochrome c release. This event was absent in the taxol-resistant cells.
Figures

Similar articles
-
Dimerization of mitochondrial Bax is associated with increased drug response in Bax-transfected A253 cells.Oncol Res. 1999;11(2):91-9. Oncol Res. 1999. PMID: 10489165
-
Cisplatin-induced apoptosis proceeds by caspase-3-dependent and -independent pathways in cisplatin-resistant and -sensitive human ovarian cancer cell lines.Cancer Res. 1999 Jul 1;59(13):3077-83. Cancer Res. 1999. PMID: 10397248
-
Formation of nuclear Bax/p53 complexes is associated with chemotherapy induced apoptosis.Oncogene. 2000 Dec 14;19(54):6216-28. doi: 10.1038/sj.onc.1203995. Oncogene. 2000. PMID: 11175336
-
RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells involves Bax translocation to mitochondria.Cancer Res. 2003 May 15;63(10):2483-91. Cancer Res. 2003. PMID: 12750270
-
Influence of Bax or Bcl-2 overexpression on the ceramide-dependent apoptotic pathway in glioma cells.Oncogene. 2000 Jul 20;19(31):3508-20. doi: 10.1038/sj.onc.1203699. Oncogene. 2000. PMID: 10918609
Cited by
-
Myxoma virus M11L blocks apoptosis through inhibition of conformational activation of Bax at the mitochondria.J Virol. 2006 Feb;80(3):1140-51. doi: 10.1128/JVI.80.3.1140-1151.2006. J Virol. 2006. PMID: 16414991 Free PMC article.
-
Mitocans as anti-cancer agents targeting mitochondria: lessons from studies with vitamin E analogues, inhibitors of complex II.J Bioenerg Biomembr. 2007 Feb;39(1):65-72. doi: 10.1007/s10863-006-9060-z. J Bioenerg Biomembr. 2007. PMID: 17294131 Review.
-
Commitment to apoptosis in CD4(+) T lymphocytes productively infected with human immunodeficiency virus type 1 is initiated by lysosomal membrane permeabilization, itself induced by the isolated expression of the viral protein Nef.J Virol. 2007 Oct;81(20):11426-40. doi: 10.1128/JVI.00597-07. Epub 2007 Aug 1. J Virol. 2007. PMID: 17670831 Free PMC article.
-
Zerumbone-Loaded Nanostructured Lipid Carrier Induces Apoptosis of Canine Mammary Adenocarcinoma Cells.Biomed Res Int. 2018 Oct 15;2018:8691569. doi: 10.1155/2018/8691569. eCollection 2018. Biomed Res Int. 2018. PMID: 30410940 Free PMC article.
-
ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL.EMBO J. 2007 Jun 20;26(12):2856-67. doi: 10.1038/sj.emboj.7601723. Epub 2007 May 24. EMBO J. 2007. PMID: 17525735 Free PMC article.
References
-
- Adams J.M. and Cory,S. (1998) The Bcl-2 protein family: arbiters of cell survival. Science, 281, 1322–1326. - PubMed
-
- Antonsson B. et al. (1997) Inhibition of Bax channel-forming activity by Bcl-2. Science, 277, 370–372. - PubMed
-
- Antonsson B., Montessuit,S., Sanchez,B. and Martinou,J.-C. (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem., 276, 11615–11623. - PubMed
-
- Bouillet P., Metcalf,D., Huang,D.C., Tarlington,D.M., Kay,T.W., Kontgen,F., Adams,J.M. and Strasser,A. (1999) Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostatsis and to preclude autoimmunity. Science, 286, 1735–1738. - PubMed
-
- Carethers J.M. and Pham,T.T. (2000) Mutations of transforming growth factor β1 type II receptor, Bax and insulin like growth factor II receptor genes in microsatellite unstable cell lines. In Vivo, 14, 13–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

