Crystal structures of two human pyrophosphorylase isoforms in complexes with UDPGlc(Gal)NAc: role of the alternatively spliced insert in the enzyme oligomeric assembly and active site architecture
- PMID: 11707391
- PMCID: PMC125729
- DOI: 10.1093/emboj/20.22.6191
Crystal structures of two human pyrophosphorylase isoforms in complexes with UDPGlc(Gal)NAc: role of the alternatively spliced insert in the enzyme oligomeric assembly and active site architecture
Abstract
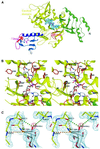
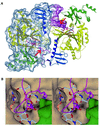
The recently published human genome with its relatively modest number of genes has highlighted the importance of post-transcriptional and post-translational modifications, such as alternative splicing or glycosylation, in generating the complexities of human biology. The human UDP-N-acetylglucosamine (UDPGlcNAc) pyrophosphorylases AGX1 and AGX2, which differ in sequence by an alternatively spliced 17 residue peptide, are key enzymes synthesizing UDPGlcNAc, an essential precursor for protein glycosylation. To better understand the catalytic mechanism of these enzymes and the role of the alternatively spliced segment, we have solved the crystal structures of AGX1 and AGX2 in complexes with UDPGlcNAc (at 1.9 and 2.4 A resolution, respectively) and UDPGalNAc (at 2.2 and 2.3 A resolution, respectively). Comparison with known structures classifies AGX1 and AGX2 as two new members of the SpsA-GnT I Core superfamily and, together with mutagenesis analysis, helps identify residues critical for catalysis. Most importantly, our combined structural and biochemical data provide evidence for a change in the oligomeric assembly accompanied by a significant modification of the active site architecture, a result suggesting that the two isoforms generated by alternative splicing may have distinct catalytic properties.
Figures
Similar articles
-
Discovery of Novel Inhibitors Targeting Multi-UDP-hexose Pyrophosphorylases as Anticancer Agents.Molecules. 2020 Feb 3;25(3):645. doi: 10.3390/molecules25030645. Molecules. 2020. PMID: 32028604 Free PMC article.
-
Structural analysis of the H166G site-directed mutant of galactose-1-phosphate uridylyltransferase complexed with either UDP-glucose or UDP-galactose: detailed description of the nucleotide sugar binding site.Biochemistry. 1997 Feb 11;36(6):1212-22. doi: 10.1021/bi9626517. Biochemistry. 1997. PMID: 9063869
-
X-ray crystal structure of rabbit N-acetylglucosaminyltransferase I: catalytic mechanism and a new protein superfamily.EMBO J. 2000 Oct 16;19(20):5269-80. doi: 10.1093/emboj/19.20.5269. EMBO J. 2000. PMID: 11032794 Free PMC article.
-
Genetic approaches to biochemical questions: insights into the functional requirements of proline 185 in the active site of human galactose-1-phosphate uridylyltransferase.SAAS Bull Biochem Biotechnol. 1997;10:43-8. SAAS Bull Biochem Biotechnol. 1997. PMID: 9274061 Review.
-
Structural and molecular biology of type I galactosemia: disease-associated mutations.IUBMB Life. 2011 Nov;63(11):949-54. doi: 10.1002/iub.510. Epub 2011 Sep 30. IUBMB Life. 2011. PMID: 21960482 Review.
Cited by
-
Substrate Specificity and Inhibitor Sensitivity of Plant UDP-Sugar Producing Pyrophosphorylases.Front Plant Sci. 2017 Sep 20;8:1610. doi: 10.3389/fpls.2017.01610. eCollection 2017. Front Plant Sci. 2017. PMID: 28970843 Free PMC article.
-
Expression and Bioinformatics-Based Functional Analysis of UAP1 in Lung Adenocarcinoma.Cancer Manag Res. 2020 Nov 25;12:12111-12121. doi: 10.2147/CMAR.S282238. eCollection 2020. Cancer Manag Res. 2020. PMID: 33269005 Free PMC article.
-
Structure of uridine diphosphate N-acetylglucosamine pyrophosphorylase from Entamoeba histolytica.Acta Crystallogr F Struct Biol Commun. 2015 May;71(Pt 5):560-5. doi: 10.1107/S2053230X1500179X. Epub 2015 Apr 21. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 25945709 Free PMC article.
-
Action of Dicumarol on Glucosamine-1-Phosphate Acetyltransferase of GlmU and Mycobacterium tuberculosis.Front Microbiol. 2019 Aug 20;10:1799. doi: 10.3389/fmicb.2019.01799. eCollection 2019. Front Microbiol. 2019. PMID: 31481936 Free PMC article.
-
Detecting coevolution in and among protein domains.PLoS Comput Biol. 2007 Nov;3(11):e211. doi: 10.1371/journal.pcbi.0030211. Epub 2007 Sep 18. PLoS Comput Biol. 2007. PMID: 17983264 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

