A novel plant kinesin-related protein specifically associates with the phragmoplast organelles
- PMID: 11701879
- PMCID: PMC139462
- DOI: 10.1105/tpc.010225
A novel plant kinesin-related protein specifically associates with the phragmoplast organelles
Abstract
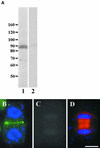
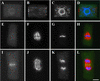
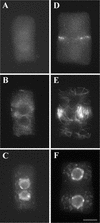
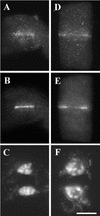
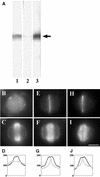
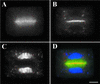
In higher plants, the formation of the cell plate during cytokinesis requires coordinated microtubule (MT) reorganization and vesicle transport in the phragmoplast. MT-based kinesin motors are important players in both processes. To understand the mechanisms underlying plant cytokinesis, we have identified AtPAKRP2 (for Arabidopsis thaliana phragmoplast-associated kinesin-related protein 2). AtPAKRP2 is an ungrouped N-terminal motor kinesin. It first appeared in a punctate pattern among interzonal MTs during late anaphase. When the phragmoplast MT array appeared in a mirror pair, AtPAKRP2 became more concentrated near the division site, and additional signal could be detected elsewhere in the phragmoplast. In contrast, the previously identified AtPAKRP1 protein is associated specifically with bundles of MTs in the phragmoplast at or near their plus ends. Localization of the tobacco homolog(s) of AtPAKRP2 was altered by treatment of brefeldin A in BY-2 cells. We discuss the possibility that AtPAKRP1 plays a role in establishing and/or maintaining the phragmoplast MT array, and AtPAKRP2 may contribute to the transport of Golgi-derived vesicles in the phragmoplast.
Figures



Similar articles
-
Identification of a phragmoplast-associated kinesin-related protein in higher plants.Curr Biol. 2000 Jun 29;10(13):797-800. doi: 10.1016/s0960-9822(00)00564-9. Curr Biol. 2000. PMID: 10898978
-
Interaction of antiparallel microtubules in the phragmoplast is mediated by the microtubule-associated protein MAP65-3 in Arabidopsis.Plant Cell. 2011 Aug;23(8):2909-23. doi: 10.1105/tpc.110.078204. Epub 2011 Aug 26. Plant Cell. 2011. PMID: 21873565 Free PMC article.
-
Localization of two homologous Arabidopsis kinesin-related proteins in the phragmoplast.Planta. 2004 Nov;220(1):156-64. doi: 10.1007/s00425-004-1324-4. Epub 2004 Jul 16. Planta. 2004. PMID: 15258761
-
The rise and fall of the phragmoplast microtubule array.Curr Opin Plant Biol. 2013 Dec;16(6):757-63. doi: 10.1016/j.pbi.2013.10.008. Epub 2013 Oct 27. Curr Opin Plant Biol. 2013. PMID: 24172707 Review.
-
Roles for kinesin and myosin during cytokinesis.Philos Trans R Soc Lond B Biol Sci. 2002 Jun 29;357(1422):761-6. doi: 10.1098/rstb.2002.1093. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12079671 Free PMC article. Review.
Cited by
-
Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins.Protoplasma. 2012 Oct;249(4):887-99. doi: 10.1007/s00709-011-0343-9. Epub 2011 Oct 25. Protoplasma. 2012. PMID: 22038119 Review.
-
A plant-specific kinesin binds to actin microfilaments and interacts with cortical microtubules in cotton fibers.Plant Physiol. 2004 Dec;136(4):3945-55. doi: 10.1104/pp.104.052340. Epub 2004 Nov 19. Plant Physiol. 2004. PMID: 15557092 Free PMC article.
-
The {gamma}-tubulin complex protein GCP4 is required for organizing functional microtubule arrays in Arabidopsis thaliana.Plant Cell. 2010 Jan;22(1):191-204. doi: 10.1105/tpc.109.071191. Epub 2010 Jan 29. Plant Cell. 2010. PMID: 20118227 Free PMC article.
-
The functions of kinesin and kinesin-related proteins in eukaryotes.Cell Adh Migr. 2020 Dec;14(1):139-152. doi: 10.1080/19336918.2020.1810939. Cell Adh Migr. 2020. PMID: 32842864 Free PMC article. Review.
-
MICROTUBULE-ASSOCIATED PROTEIN65 is essential for maintenance of phragmoplast bipolarity and formation of the cell plate in Physcomitrella patens.Plant Cell. 2013 Nov;25(11):4479-92. doi: 10.1105/tpc.113.117432. Epub 2013 Nov 22. Plant Cell. 2013. PMID: 24272487 Free PMC article.
References
-
- Asada, T., Sonobe, S., and Shibaoka, H. (1991). Microtubule translocation in the cytokinetic apparatus of cultured tobacco cells. Nature 350 238–241.
-
- Asada, T., Kuriyama, R., and Shibaoka, H. (1997). TKRP125, a kinesin-related protein involved in the centrosome-independent organization of the cytokinetic apparatus in tobacco BY-2 cells. J. Cell Sci. 110 179–189. - PubMed
-
- Barroso, C., Chan, J., Allan, V., Doonan, J., Hussey, P., and Lloyd, C. (2000). Two kinesin-related proteins associated with the cold-stable cytoskeleton of carrot cells: Characterization of a novel kinesin, DcKRP120-2. Plant J. 24 859–868. - PubMed
-
- Bowser, J., and Reddy, A.S.N. (1997). Localization of a kinesin-like calmodulin-binding protein in dividing cells of Arabidopsis and tobacco. Plant J. 12 1429–1437. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

