Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses
- PMID: 11679158
- PMCID: PMC3401017
- DOI: 10.1089/088922201753197123
Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses
Abstract
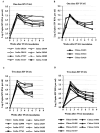
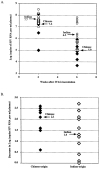
The purpose of this study was to determine whether rhesus monkeys of Chinese origin are suitable for studies of mucosal lentivirus transmission by comparing the relative ability of these animals and rhesus macaques of Indian origin to become infected by vaginal (IVAG) inoculation with SIVmac251. In addition, we sought to test the hypothesis that differences in viral load during the first few weeks after inoculation were due to the relative strength of the anti-SIV immune responses in the two populations of rhesus macaques. Significant difference was not observed between the number of Indian and Chinese origin monkeys that were infected after IVAG SIV inoculation in this study. For 8-9 weeks after infection there was considerable overlap in the range of viral loads among the Indian and Chinese animals and the variation among the Indian origin animals was greater than the variation among the Chinese origin monkeys. By 6 weeks postinfection, viral loads in SIV-infected Chinese origin monkeys tended to be at the lower end of the range of viral loads observed in SIV-infected Indian origin monkeys. The strength of the anti-SIV antibody response was also more variable in the Indian origin rhesus macaques, but at 6-8 weeks postinfection, Chinese and Indian origin rhesus macaques had similar titers of anti-SIV antibodies. Microsatellite allele frequencies differed between Chinese and Indian rhesus macaques; however, the majority of alleles present in Indian-origin animals were also found in Chinese macaques. Together these results show that host factors, other than geographic origin, determine the ability of a rhesus macaque to be infected after IVAG SIV exposure and that geographic origin does not predict the viral load of SIV-infected animals during the first 8-9 weeks after IVAG inoculation.
Figures
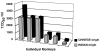
Similar articles
-
Route of simian immunodeficiency virus inoculation determines the complexity but not the identity of viral variant populations that infect rhesus macaques.J Virol. 2001 Apr;75(8):3753-65. doi: 10.1128/JVI.75.8.3753-3765.2001. J Virol. 2001. PMID: 11264364 Free PMC article.
-
SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans.AIDS. 2002 Jul 26;16(11):1489-96. doi: 10.1097/00002030-200207260-00005. AIDS. 2002. PMID: 12131186
-
Species-specific variation in SIV disease progression between Chinese and Indian subspecies of rhesus macaque.J Med Primatol. 2002 Aug;31(4-5):171-8. doi: 10.1034/j.1600-0684.2002.02003.x. J Med Primatol. 2002. PMID: 12390539
-
Localization of Simian immunodeficiency virus-infected cells in the genital tract of male and female Rhesus macaques.J Reprod Immunol. 1998 Dec;41(1-2):331-9. doi: 10.1016/s0165-0378(98)00069-2. J Reprod Immunol. 1998. PMID: 10213321 Review.
-
SIV infection of rhesus macaques of Chinese origin: a suitable model for HIV infection in humans.Retrovirology. 2013 Aug 15;10:89. doi: 10.1186/1742-4690-10-89. Retrovirology. 2013. PMID: 23947613 Free PMC article. Review.
Cited by
-
Infection of Chinese Rhesus Monkeys with a Subtype C SHIV Resulted in Attenuated In Vivo Viral Replication Despite Successful Animal-to-Animal Serial Passages.Viruses. 2021 Mar 2;13(3):397. doi: 10.3390/v13030397. Viruses. 2021. PMID: 33801437 Free PMC article.
-
CD8+-cell-mediated suppression of virulent simian immunodeficiency virus during tenofovir treatment.J Virol. 2004 May;78(10):5324-37. doi: 10.1128/jvi.78.10.5324-5337.2004. J Virol. 2004. PMID: 15113912 Free PMC article.
-
Comparison of immunogenicity and safety outcomes of a malaria vaccine FMP013/ALFQ in rhesus macaques (Macaca mulatta) of Indian and Chinese origin.Malar J. 2019 Nov 27;18(1):377. doi: 10.1186/s12936-019-3014-5. Malar J. 2019. PMID: 31775762 Free PMC article.
-
Vaccination of rhesus macaques with a vif-deleted simian immunodeficiency virus proviral DNA vaccine.Virology. 2008 May 10;374(2):261-72. doi: 10.1016/j.virol.2008.01.020. Epub 2008 Feb 7. Virology. 2008. PMID: 18261756 Free PMC article.
-
High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection.J Virol. 2009 Apr;83(7):3288-97. doi: 10.1128/JVI.02423-08. Epub 2009 Jan 7. J Virol. 2009. PMID: 19129448 Free PMC article.
References
-
- Joag SV, Stephens EB, Adams RJ, Foresman L, Narayan O. Pathogenesis of SIVmac infection in Chinese and Indian rhesus macaques: Effects of splenectomy on virus burden. Virology. 1994;200:436–446. - PubMed
-
- Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources. Guide for Care and Use of Laboratory Animals. National Resource Council; Washington, D.C.: 1998.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

