Coilin forms the bridge between Cajal bodies and SMN, the spinal muscular atrophy protein
- PMID: 11641277
- PMCID: PMC312817
- DOI: 10.1101/gad.908401
Coilin forms the bridge between Cajal bodies and SMN, the spinal muscular atrophy protein
Abstract
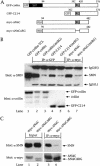
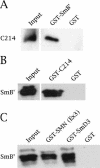
Spinal muscular atrophy (SMA) is a genetic disorder caused by mutations in the human survival of motor neuron 1 gene, SMN1. SMN protein is part of a large complex that is required for biogenesis of various small nuclear ribonucleoproteins (snRNPs). Here, we report that SMN interacts directly with the Cajal body signature protein, coilin, and that this interaction mediates recruitment of the SMN complex to Cajal bodies. Mutation or deletion of specific RG dipeptide residues within coilin inhibits the interaction both in vivo and in vitro. Interestingly, GST-pulldown experiments show that coilin also binds directly to SmB'. Competition studies show that coilin competes with SmB' for binding sites on SMN. Ectopic expression of SMN and coilin constructs in mouse embryonic fibroblasts lacking endogenous coilin confirms that recruitment of SMN and splicing snRNPs to Cajal bodies depends on the coilin C-terminal RG motif. A cardinal feature of SMA patient cells is a defect in the targeting of SMN to nuclear foci; our results uncover a role for coilin in this process.
Figures

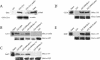


Similar articles
-
Residual Cajal bodies in coilin knockout mice fail to recruit Sm snRNPs and SMN, the spinal muscular atrophy gene product.J Cell Biol. 2001 Jul 23;154(2):293-307. doi: 10.1083/jcb.200104083. J Cell Biol. 2001. PMID: 11470819 Free PMC article.
-
Gemin proteins are required for efficient assembly of Sm-class ribonucleoproteins.Proc Natl Acad Sci U S A. 2005 Nov 29;102(48):17372-7. doi: 10.1073/pnas.0508947102. Epub 2005 Nov 21. Proc Natl Acad Sci U S A. 2005. PMID: 16301532 Free PMC article.
-
Distinct domains of the spinal muscular atrophy protein SMN are required for targeting to Cajal bodies in mammalian cells.J Cell Sci. 2006 Feb 15;119(Pt 4):680-92. doi: 10.1242/jcs.02782. Epub 2006 Jan 31. J Cell Sci. 2006. PMID: 16449324
-
The Cajal body.Biochim Biophys Acta. 2008 Nov;1783(11):2108-15. doi: 10.1016/j.bbamcr.2008.07.016. Epub 2008 Aug 3. Biochim Biophys Acta. 2008. PMID: 18755223 Review.
-
RNA splicing: more clues from spinal muscular atrophy.Curr Biol. 1999 Feb 25;9(4):R140-2. doi: 10.1016/s0960-9822(99)80083-9. Curr Biol. 1999. PMID: 10074419 Review.
Cited by
-
Ultrastructural characterization of peripheral denervation in a mouse model of Type III spinal muscular atrophy.J Neural Transm (Vienna). 2021 Jun;128(6):771-791. doi: 10.1007/s00702-021-02353-9. Epub 2021 May 17. J Neural Transm (Vienna). 2021. PMID: 33999256 Free PMC article.
-
Coilin: The first 25 years.RNA Biol. 2015;12(6):590-6. doi: 10.1080/15476286.2015.1034923. RNA Biol. 2015. PMID: 25970135 Free PMC article. Review.
-
Phosphorylation and the Cajal body: modification in search of function.Arch Biochem Biophys. 2010 Apr 15;496(2):69-76. doi: 10.1016/j.abb.2010.02.012. Epub 2010 Mar 1. Arch Biochem Biophys. 2010. PMID: 20193656 Free PMC article. Review.
-
The Cajal body: a meeting place for spliceosomal snRNPs in the nuclear maze.Chromosoma. 2006 Oct;115(5):343-54. doi: 10.1007/s00412-006-0056-6. Epub 2006 Mar 31. Chromosoma. 2006. PMID: 16575476 Review.
-
Fibroblast growth factor-2(23) binds directly to the survival of motoneuron protein and is associated with small nuclear RNAs.Biochem J. 2004 Dec 15;384(Pt 3):559-65. doi: 10.1042/BJ20040801. Biochem J. 2004. PMID: 15222879 Free PMC article.
References
-
- Brahms H, Raymackers J, Union A, de Keyser F, Meheus L, Lührmann R. The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form a major B-cell epitope for anti-Sm autoantibodies. J Biol Chem. 2000;275:17122–17129. - PubMed
-
- Bühler D, Raker V, Lührmann R, Fischer U. Essential role for the Tudor domain of SMN in spliceosomal U snRNP assembly: Implications for spinal muscular atrophy. Hum Mol Genet. 1999;8:2351–2357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
