A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell
- PMID: 11606735
- PMCID: PMC60822
- DOI: 10.1073/pnas.221381398
A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell
Erratum in
- Proc Natl Acad Sci U S A 2001 Dec 4;98(25):14744
Abstract
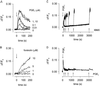
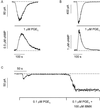
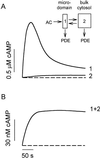
cAMP, the classical second messenger, regulates many diverse cellular functions. The primary effector of cAMP signals, protein kinase A, differentially phosphorylates hundreds of cellular targets. Little is known, however, about the spatial and temporal nature of cAMP signals and their information content. Thus, it is largely unclear how cAMP, in response to different stimuli, orchestrates such a wide variety of cellular responses. Previously, we presented evidence that cAMP is produced in subcellular compartments near the plasma membrane, and that diffusion of cAMP from these compartments to the bulk cytosol is hindered. Here we report that a uniform extracellular stimulus initiates distinct cAMP signals within different cellular compartments. By using cyclic nucleotide-gated ion channels engineered as cAMP biosensors, we found that prostaglandin E(1) stimulation of human embryonic kidney cells caused a transient increase in cAMP concentration near the membrane. Interestingly, in the same time frame, the total cellular cAMP rose to a steady level. The decline in cAMP levels near the membrane was prevented by pretreatment with phosphodiesterase inhibitors. These data demonstrate that spatially and temporally distinct cAMP signals can coexist within simple cells.
Figures
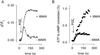
Comment in
-
The many dimensions of cAMP signaling.Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13482-4. doi: 10.1073/pnas.251533998. Proc Natl Acad Sci U S A. 2001. PMID: 11717418 Free PMC article. Review. No abstract available.
Similar articles
-
Resolution of cAMP signals in three-dimensional microdomains using novel, real-time sensors.Proc West Pharmacol Soc. 2004;47:1-5. Proc West Pharmacol Soc. 2004. PMID: 15633600
-
Cellular mechanisms underlying prostaglandin-induced transient cAMP signals near the plasma membrane of HEK-293 cells.Am J Physiol Cell Physiol. 2007 Jan;292(1):C319-31. doi: 10.1152/ajpcell.00121.2006. Epub 2006 Aug 9. Am J Physiol Cell Physiol. 2007. PMID: 16899551 Free PMC article.
-
Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion.J Gen Physiol. 2000 Aug;116(2):147-61. doi: 10.1085/jgp.116.2.147. J Gen Physiol. 2000. PMID: 10919863 Free PMC article.
-
Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases.FEBS J. 2009 Apr;276(7):1790-9. doi: 10.1111/j.1742-4658.2009.06926.x. Epub 2009 Feb 23. FEBS J. 2009. PMID: 19243430 Review.
-
Review article: cyclic AMP sensors in living cells: what signals can they actually measure?Ann Biomed Eng. 2002 Sep;30(8):1088-99. doi: 10.1114/1.1511242. Ann Biomed Eng. 2002. PMID: 12449769 Review.
Cited by
-
Prominin-1-Radixin axis controls hepatic gluconeogenesis by regulating PKA activity.EMBO Rep. 2020 Nov 5;21(11):e49416. doi: 10.15252/embr.201949416. Epub 2020 Oct 8. EMBO Rep. 2020. PMID: 33030802 Free PMC article.
-
Cell specific dopamine modulation of the transient potassium current in the pyloric network by the canonical D1 receptor signal transduction cascade.J Neurophysiol. 2010 Aug;104(2):873-84. doi: 10.1152/jn.00195.2010. Epub 2010 Jun 2. J Neurophysiol. 2010. PMID: 20519576 Free PMC article.
-
Inactivation of the Carney complex gene 1 (PRKAR1A) alters spatiotemporal regulation of cAMP and cAMP-dependent protein kinase: a study using genetically encoded FRET-based reporters.Hum Mol Genet. 2014 Mar 1;23(5):1163-74. doi: 10.1093/hmg/ddt510. Epub 2013 Oct 10. Hum Mol Genet. 2014. PMID: 24122441 Free PMC article.
-
Diffusion in cytoplasm: effects of excluded volume due to internal membranes and cytoskeletal structures.Biophys J. 2009 Aug 5;97(3):758-67. doi: 10.1016/j.bpj.2009.05.036. Biophys J. 2009. PMID: 19651034 Free PMC article.
-
Heterogeneity of pulmonary endothelial cyclic nucleotide response to Pseudomonas aeruginosa ExoY infection.Am J Physiol Lung Cell Mol Physiol. 2015 Nov 15;309(10):L1199-207. doi: 10.1152/ajplung.00165.2015. Epub 2015 Sep 18. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26386118 Free PMC article.
References
-
- Sunahara R K, Dessauer C W, Gilman A G. Annu Rev Pharmacol Toxicol. 1996;36:461–480. - PubMed
-
- Beavo J A. Physiol Rev. 1995;75:725–748. - PubMed
-
- Walsh D A, Perkins J P, Krebs E G. J Biol Chem. 1968;243:3763–3765. - PubMed
-
- Gray P C, Scott J D, Catterall W A. Curr Opin Neurobiol. 1998;8:330–334. - PubMed
-
- Francis S, Corbin J D. Crit Rev Clin Lab Sci. 1999;36:275–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

