Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide
- PMID: 11606325
- PMCID: PMC1573017
- DOI: 10.1038/sj.bjp.0704327
Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide
Abstract
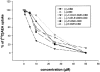
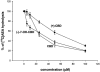
1. (-)-Cannabidiol (CBD) is a non-psychotropic component of Cannabis with possible therapeutic use as an anti-inflammatory drug. Little is known on the possible molecular targets of this compound. We investigated whether CBD and some of its derivatives interact with vanilloid receptor type 1 (VR1), the receptor for capsaicin, or with proteins that inactivate the endogenous cannabinoid, anandamide (AEA). 2. CBD and its enantiomer, (+)-CBD, together with seven analogues, obtained by exchanging the C-7 methyl group of CBD with a hydroxy-methyl or a carboxyl function and/or the C-5' pentyl group with a di-methyl-heptyl (DMH) group, were tested on: (a) VR1-mediated increase in cytosolic Ca(2+) concentrations in cells over-expressing human VR1; (b) [(14)C]-AEA uptake by RBL-2H3 cells, which is facilitated by a selective membrane transporter; and (c) [(14)C]-AEA hydrolysis by rat brain membranes, which is catalysed by the fatty acid amide hydrolase. 3. Both CBD and (+)-CBD, but not the other analogues, stimulated VR1 with EC(50)=3.2 - 3.5 microM, and with a maximal effect similar in efficacy to that of capsaicin, i.e. 67 - 70% of the effect obtained with ionomycin (4 microM). CBD (10 microM) desensitized VR1 to the action of capsaicin. The effects of maximal doses of the two compounds were not additive. 4. (+)-5'-DMH-CBD and (+)-7-hydroxy-5'-DMH-CBD inhibited [(14)C]-AEA uptake (IC(50)=10.0 and 7.0 microM); the (-)-enantiomers were slightly less active (IC(50)=14.0 and 12.5 microM). 5. CBD and (+)-CBD were also active (IC(50)=22.0 and 17.0 microM). CBD (IC(50)=27.5 microM), (+)-CBD (IC(50)=63.5 microM) and (-)-7-hydroxy-CBD (IC(50)=34 microM), but not the other analogues (IC(50)>100 microM), weakly inhibited [(14)C]-AEA hydrolysis. 6. Only the (+)-isomers exhibited high affinity for CB(1) and/or CB(2) cannabinoid receptors. 7. These findings suggest that VR1 receptors, or increased levels of endogenous AEA, might mediate some of the pharmacological effects of CBD and its analogues. In view of the facile high yield synthesis, and the weak affinity for CB(1) and CB(2) receptors, (-)-5'-DMH-CBD represents a valuable candidate for further investigation as inhibitor of AEA uptake and a possible new therapeutic agent.
Figures
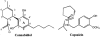
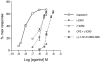
Similar articles
-
Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity.FEBS Lett. 2000 Oct 13;483(1):52-6. doi: 10.1016/s0014-5793(00)02082-2. FEBS Lett. 2000. PMID: 11033355
-
Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors.Biochem Biophys Res Commun. 1999 Aug 19;262(1):275-84. doi: 10.1006/bbrc.1999.1105. Biochem Biophys Res Commun. 1999. PMID: 10448105
-
The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism.J Biol Chem. 2001 Apr 20;276(16):12856-63. doi: 10.1074/jbc.M008555200. Epub 2001 Jan 26. J Biol Chem. 2001. PMID: 11278420
-
Anandamide receptors.Prostaglandins Leukot Essent Fatty Acids. 2002 Feb-Mar;66(2-3):377-91. doi: 10.1054/plef.2001.0349. Prostaglandins Leukot Essent Fatty Acids. 2002. PMID: 12052051 Review.
-
New perspectives on enigmatic vanilloid receptors.Trends Neurosci. 2000 Oct;23(10):491-7. doi: 10.1016/s0166-2236(00)01630-1. Trends Neurosci. 2000. PMID: 11006466 Review.
Cited by
-
Effects of Cannabidiol (CBD) on Doxorubicin-Induced Anxiety and Depression-like Behaviors and mRNA Expression of Inflammatory Markers in Rats.Brain Sci. 2024 Sep 30;14(10):999. doi: 10.3390/brainsci14100999. Brain Sci. 2024. PMID: 39452013 Free PMC article.
-
The Healthy and Diseased Retina Seen through Neuron-Glia Interactions.Int J Mol Sci. 2024 Jan 17;25(2):1120. doi: 10.3390/ijms25021120. Int J Mol Sci. 2024. PMID: 38256192 Free PMC article. Review.
-
Cannabidiolic acid, a major cannabinoid in fiber-type cannabis, is an inhibitor of MDA-MB-231 breast cancer cell migration.Toxicol Lett. 2012 Nov 15;214(3):314-9. doi: 10.1016/j.toxlet.2012.08.029. Epub 2012 Sep 8. Toxicol Lett. 2012. PMID: 22963825 Free PMC article.
-
In Vitro Evidence of Selective Pro-Apoptotic Action of the Pure Cannabidiol and Cannabidiol-Rich Extract.Molecules. 2023 Dec 1;28(23):7887. doi: 10.3390/molecules28237887. Molecules. 2023. PMID: 38067615 Free PMC article.
-
Development and Characterization of a Microemulsion Containing a Cannabidiol Oil and a Hydrophilic Extract from Sambucus ebulus for Topical Administration.Pharmaceutics. 2024 May 24;16(6):705. doi: 10.3390/pharmaceutics16060705. Pharmaceutics. 2024. PMID: 38931831 Free PMC article.
References
-
- AGURELL S., HALLDIN M., LINDGREN J.E., OHLSSON A., WIDMAN M., GILLESPIE H., HOLLISTER L. Pharmacokinetics and metabolism of delta-1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol. Rev. 1986;38:21–43. - PubMed
-
- BAEK S.H., SREBNIK M., MECHOULAM R. Borontrifluoride on alumina–a modified Lewis acid reagent. An improved synthesis of cannabidiol. Tetrahedron Lett. 1985;26:1083–1086.
-
- BAYEWITCH M., RHEE M.H., AVIDOR-REISS T., BREUER A., MECHOULAM R., VOGEL Z. (−)-Delta9-tetrahydrocannabinol antagonizes the peripheral cannabinoid receptor-mediated inhibition of adenylyl cyclase. J. Biol. Chem. 1996;271:9902–9905. - PubMed
-
- BISOGNO T., MAURELLI S., MELCK D., DE PETROCELLIS L., DI MARZO V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J. Biol. Chem. 1997;272:3315–3323. - PubMed
-
- BORNHEIM L.M., CORREIA M.A. Effect of cannabidiol on cytochrome P-450 isozymes. Biochem. Pharmacol. 1989;38:2789–2794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

