Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge
- PMID: 11602747
- PMCID: PMC114687
- DOI: 10.1128/JVI.75.22.11079-11087.2001
Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge
Abstract
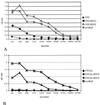
Foreign glycoproteins expressed in recombinant vesicular stomatitis virus (VSV) can elicit specific and protective immunity in the mouse model. We have previously demonstrated the expression of respiratory syncytial virus (RSV) G (attachment) and F (fusion) glycoprotein genes in recombinant VSV. In this study, we demonstrate the expression of RSV F and G glycoproteins in attenuated, nonpropagating VSVs which lack the VSV G gene (VSVDeltaG) and the incorporation of these RSV proteins into recombinant virions. We also show that intranasal vaccination of mice with nondefective VSV recombinants expressing RSV G (VSV-RSV G) or RSV F (VSV-RSV F) elicited RSV-specific antibodies in serum (by enzyme-linked immunosorbent assay [ELISA]) as well as neutralizing antibodies to RSV and afford complete protection against RSV challenge. In contrast, VSVDeltaG-RSV F induced detectable serum antibodies to RSV by ELISA, but no detectable neutralizing antibodies, yet it still protected from RSV challenge. VSVDeltaG-RSV G failed to induce any detectable serum (by ELISA) or neutralizing antibodies and failed to protect from RSV challenge. The attenuated, nonpropagating VSVDeltaG-RSV F is a particularly attractive candidate for a live attenuated recombinant RSV vaccine.
Figures



Similar articles
-
Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3.J Virol. 2001 May;75(10):4594-603. doi: 10.1128/JVI.75.10.4594-4603.2001. J Virol. 2001. PMID: 11312329 Free PMC article.
-
Attenuated Human Parainfluenza Virus Type 1 (HPIV1) Expressing the Fusion Glycoprotein of Human Respiratory Syncytial Virus (RSV) as a Bivalent HPIV1/RSV Vaccine.J Virol. 2015 Oct;89(20):10319-32. doi: 10.1128/JVI.01380-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223633 Free PMC article.
-
Chikungunya, Influenza, Nipah, and Semliki Forest Chimeric Viruses with Vesicular Stomatitis Virus: Actions in the Brain.J Virol. 2017 Feb 28;91(6):e02154-16. doi: 10.1128/JVI.02154-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077641 Free PMC article.
-
Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection.Pediatr Infect Dis J. 2004 Jan;23(1 Suppl):S46-57. doi: 10.1097/01.inf.0000108192.94692.d2. Pediatr Infect Dis J. 2004. PMID: 14730270 Review.
-
Respiratory syncytial virus infection in older persons.Vaccine. 1998 Nov;16(18):1775-8. doi: 10.1016/s0264-410x(98)00142-x. Vaccine. 1998. PMID: 9778756 Review.
Cited by
-
Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus.J Virol. 2007 Jun;81(11):5749-58. doi: 10.1128/JVI.02835-06. Epub 2007 Mar 28. J Virol. 2007. PMID: 17392369 Free PMC article.
-
rVSVΔG-ZEBOV-GP (also designated V920) recombinant vesicular stomatitis virus pseudotyped with Ebola Zaire Glycoprotein: Standardized template with key considerations for a risk/benefit assessment.Vaccine X. 2019 Jan 29;1:100009. doi: 10.1016/j.jvacx.2019.100009. eCollection 2019 Apr 11. Vaccine X. 2019. PMID: 31384731 Free PMC article.
-
RSV Vaccine Based on Rhabdoviral Vector Protects after Single Immunization.Vaccines (Basel). 2019 Jul 3;7(3):59. doi: 10.3390/vaccines7030059. Vaccines (Basel). 2019. PMID: 31277325 Free PMC article.
-
Live virus vaccines based on a vesicular stomatitis virus (VSV) backbone: Standardized template with key considerations for a risk/benefit assessment.Vaccine. 2016 Dec 12;34(51):6597-6609. doi: 10.1016/j.vaccine.2016.06.071. Epub 2016 Jul 6. Vaccine. 2016. PMID: 27395563 Free PMC article. Review.
-
Acute reactogenicity after intramuscular immunization with recombinant vesicular stomatitis virus is linked to production of IL-1β.PLoS One. 2012;7(10):e46516. doi: 10.1371/journal.pone.0046516. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056330 Free PMC article.
References
-
- Abman S H, Ogle J W, Butler-Simon N, Rumack C M, Accurso F J. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J Pediatr. 1988;113:826–830. - PubMed
-
- Anonymous. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537. - PubMed
-
- Belshe R B, Anderson E L, Walsh E E. Immunogenicity of purified F glycoprotein of respiratory syncytial virus: clinical and immune responses to subsequent natural infection in children. J Infect Dis. 1993;168:1024–1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

