Insertions in the gG gene of pseudorabies virus reduce expression of the upstream Us3 protein and inhibit cell-to-cell spread of virus infection
- PMID: 11602726
- PMCID: PMC114666
- DOI: 10.1128/JVI.75.22.10856-10869.2001
Insertions in the gG gene of pseudorabies virus reduce expression of the upstream Us3 protein and inhibit cell-to-cell spread of virus infection
Abstract
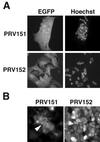
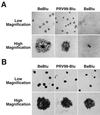
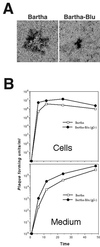
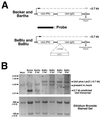
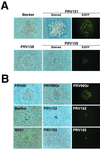
The alphaherpesvirus Us4 gene encodes glycoprotein G (gG), which is conserved in most viruses of the alphaherpesvirus subfamily. In the swine pathogen pseudorabies virus (PRV), mutant viruses with internal deletions and insertions in the gG gene have shown no discernible phenotypes. We report that insertions in the gG locus of the attenuated PRV strain Bartha show reduced virulence in vivo and are defective in their ability to spread from cell to cell in a cell-type-specific manner. Similar insertions in the gG locus of the wild-type PRV strain Becker had no effect on the ability of virus infection to spread between cells. Insertions in the gG locus of the virulent NIA-3 strain gave results similar to those found with the Bartha strain. To examine the role of gG in cell-to-cell spread, a nonsense mutation in the gG signal sequence was constructed and crossed into the Bartha strain. This mutant, PRV157, failed to express gG yet had cell-to-cell spread properties indistinguishable from those of the parental Bartha strain. These data indicated that, while insertions in the gG locus result in decreased cell-to-cell spread, the phenotype was not due to loss of gG expression as first predicted. Analysis of gene expression upstream and downstream of gG revealed that expression of the upstream Us3 protein is reduced by insertion of lacZ or egfp at the gG locus. By contrast, expression of the gene immediately downstream of gG, Us6, which encodes glycoprotein gD, was not affected by insertions in gG. These data indicate that DNA insertions in gG have polar effects and suggest that the serine/threonine kinase encoded by the Us3 gene, and not gG, functions in the spread of viral infection between cells.
Figures


Similar articles
-
Characterization of a quadruple glycoprotein-deleted pseudorabies virus mutant for use as a biologically safe live virus vaccine.J Gen Virol. 1994 Jul;75 ( Pt 7):1723-33. doi: 10.1099/0022-1317-75-7-1723. J Gen Virol. 1994. PMID: 8021601
-
The US3 Protein of Pseudorabies Virus Drives Viral Passage across the Basement Membrane in Porcine Respiratory Mucosa Explants.J Virol. 2016 Nov 14;90(23):10945-10950. doi: 10.1128/JVI.01577-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27681139 Free PMC article.
-
The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus.J Gen Virol. 1995 Jul;76 ( Pt 7):1851-9. doi: 10.1099/0022-1317-76-7-1851. J Gen Virol. 1995. PMID: 9049392
-
Pseudorabies virus infections in pigs. Role of viral proteins in virulence, pathogenesis and transmission.Vet Res. 1997;28(1):1-17. Vet Res. 1997. PMID: 9172836 Review.
-
Pseudorabies (Aujeszky's disease) virus: state of the art. August 1993.Acta Vet Hung. 1994;42(2-3):153-77. Acta Vet Hung. 1994. PMID: 7810409 Review.
Cited by
-
Transcriptome signature of virulent and attenuated pseudorabies virus-infected rodent brain.J Virol. 2006 Feb;80(4):1773-86. doi: 10.1128/JVI.80.4.1773-1786.2006. J Virol. 2006. PMID: 16439534 Free PMC article.
-
Pseudorabies virus infection alters neuronal activity and connectivity in vitro.PLoS Pathog. 2009 Oct;5(10):e1000640. doi: 10.1371/journal.ppat.1000640. Epub 2009 Oct 30. PLoS Pathog. 2009. PMID: 19876391 Free PMC article.
-
Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus.Traffic. 2008 Sep;9(9):1458-70. doi: 10.1111/j.1600-0854.2008.00782.x. Epub 2008 Jun 28. Traffic. 2008. PMID: 18564370 Free PMC article.
-
A microfluidic chamber for analysis of neuron-to-cell spread and axonal transport of an alpha-herpesvirus.PLoS One. 2008 Jun 18;3(6):e2382. doi: 10.1371/journal.pone.0002382. PLoS One. 2008. PMID: 18560518 Free PMC article.
-
Role of pseudorabies virus Us3 protein kinase during neuronal infection.J Virol. 2006 Jul;80(13):6387-98. doi: 10.1128/JVI.00352-06. J Virol. 2006. PMID: 16775327 Free PMC article.
References
-
- Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. - PubMed
-
- Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. - PubMed
-
- Bartha A, Belák S, Benyeda J. Trypsin and heat resistance of some strains of the virus group. Acta Vet Hung. 1969;19:97–99. - PubMed
-
- Bennett L M, Timmins J G, Thomsen D R, Post L E. The processing of pseudorabies virus glycoprotein gX in infected cells and in an uninfected cell line. Virology. 1986;155:707–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

